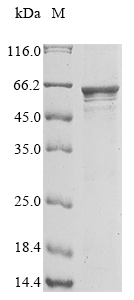
To express the recombinant human LRRC15 protein in E.coli cells, a DNA fragment encoding the human LRRC15 protein (22-538aa) is inserted into a plasmid vector along with the N-terminal 10xHis and C-terminal Myc-tag gene and transferred to the E.coli cells. Cells containing the plasmid are screened, cultured, and induced to express the LRRC15 protein. Lysing the cells allows for the collection of the recombinant human LRRC15 protein, which is purified through affinity purification and then identified through SDS-PAGE and subsequent staining of the gel with Coomassie Brilliant Blue. The purity of the recombinant human LRRC15 protein obtained is greater than 90%.
LRRC15 is a cell surface glycoprotein that plays crucial roles in various biological processes. It is normally expressed in specific tissues like the invasive cytotrophoblast layer of the placenta [1]. LRRC15 has been identified as a tumor antigen and is overexpressed in androgen-independent metastatic prostate cancer [2]. Recent studies have highlighted LRRC15's significance in cancer biology, particularly in the context of tumor immunity suppression [3]. LRRC15 is associated with cancer-associated fibroblasts (CAFs) and linked to cancer grade and outcome [4]. Moreover, LRRC15 has been implicated in viral infections, such as being a receptor for SARS-CoV-2 spike protein and influencing antiviral and antifibrotic transcriptional programs [5]. It has also been suggested that LRRC15 may have a role in controlling infection and suppressing lung fibrosis [6].
LRRC15's involvement in various diseases and its potential as a therapeutic target has garnered significant attention. Studies have explored targeting LRRC15 for inhibiting metastatic dissemination in ovarian cancer [7]. J. Purcell and his colleagues have developed LRRC15 as a stromal target for antibody-drug conjugates in cancer treatment [8]. Furthermore, LRRC15 has been proposed as a promising anti-cancer target due to its overexpression in mesenchymal-derived tumors and cancer-associated fibroblasts in the microenvironment of different types of tumors [9].
References:
[1] M. Stanbrough, G. Bubley, K. Ross, T. Golub, M. Rubin, T. Penninget al., Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer, Cancer Research, vol. 66, no. 5, p. 2815-2825, 2006. https://doi.org/10.1158/0008-5472.can-05-4000
[2] Y. Gong, Z. Wang, A. Beitelshees, C. McDonough, T. Langaee, K. Hallet al., Pharmacogenomic genome-wide meta-analysis of blood pressure response to β-blockers in hypertensive african americans, Hypertension, vol. 67, no. 3, p. 556-563, 2016. https://doi.org/10.1161/hypertensionaha.115.06345
[3] A. Krishnamurty, J. Shyer, M. Thai, V. Gandham, M. Buechler, Y. Yanget al., Lrrc15+ myofibroblasts dictate the stromal setpoint to suppress tumour immunity, Nature, vol. 611, no. 7934, p. 148-154, 2022. https://doi.org/10.1038/s41586-022-05272-1
[4] P. Baurand, J. Balland, C. Reynas, M. Ramseyer, D. Vivier, P. Bellayeet al., Development of anti-lrrc15 small fragments for imaging purposes using a phage-display scfv approach, International Journal of Molecular Sciences, vol. 23, no. 20, p. 12677, 2022. https://doi.org/10.3390/ijms232012677
[5] L. Loo, M. Waller, C. Moreno, A. Cole, A. Stella, O. Popet al., Fibroblast-expressed lrrc15 is a receptor for sars-cov-2 spike and controls antiviral and antifibrotic transcriptional programs, Plos Biology, vol. 21, no. 2, p. e3001967, 2023. https://doi.org/10.1371/journal.pbio.3001967
[6] J. Orgel, Molecular tissue responses to mechanical loading, International Journal of Molecular Sciences, vol. 23, no. 4, p. 2074, 2022. https://doi.org/10.3390/ijms23042074
[7] U. Ray, D. Jung, L. Jin, Y. Xiao, S. Dasari, S. Bhattacharyaet al., Targeting lrrc15 inhibits metastatic dissemination of ovarian cancer, Cancer Research, vol. 82, no. 6, p. 1038-1054, 2022. https://doi.org/10.1158/0008-5472.can-21-0622
[8] J. Purcell, S. Tanlimco, J. Hickson, M. Fox, M. Sho, L. Durkinet al., Lrrc15 is a novel mesenchymal protein and stromal target for antibody–drug conjugates, Cancer Research, vol. 78, no. 14, p. 4059-4072, 2018. https://doi.org/10.1158/0008-5472.can-18-0327
[9] U. Ray, C. Pathoulas, P. Thirusangu, J. Purcell, N. Kannan, & V. Shridhar, Exploiting lrrc15 as a novel therapeutic target in cancer, Cancer Research, vol. 82, no. 9, p. 1675-1681, 2022. https://doi.org/10.1158/0008-5472.can-21-3734