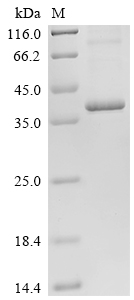
Recombinant Human STEAP1 protein is produced using an E.coli-based cell-free expression system and contains a full-length sequence from 1 to 339 amino acids. The protein features a C-terminal 10xHis-tag that helps with purification and detection. Purity exceeds 85% as verified by SDS-PAGE. With its 6 transmembrane domain structure, the protein uses a detergent platform for improved solubility and stability. This protein is intended for research applications only.
STEAP1, or Six Transmembrane Epithelial Antigen of the Prostate 1, appears to be linked to metal ion transport and reduction processes. The protein seems to play an important role in cellular metal homeostasis, particularly in reducing metal ions to their usable forms. Understanding STEAP1's function may be crucial for research focused on cellular metabolism and metal ion transport mechanisms, potentially offering insights into its involvement in various physiological and pathological processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The human STEAP1 is a complex multi-pass transmembrane protein with 6 transmembrane domains that requires proper integration into a lipid bilayer, correct disulfide bond formation, and potentially metal cofactors for its metalloreductase activity. While the in vitro E.coli system can improve solubility for membrane proteins compared to traditional E. coli expression, they still lack the native membrane environment and eukaryotic folding machinery necessary for proper multi-pass transmembrane protein folding. Without experimental validation of folding (e.g., spectroscopic structural analysis) and activity (e.g., reductase assays), the protein cannot be assumed to be correctly folded or bioactive.
1. Membrane Protein Biochemical Characterization Studies
This application is feasible for basic biophysical characterization of the STEAP1 protein (e.g., circular dichroism for secondary structure, dynamic light scattering for aggregation state). However, the data will reflect the properties of the protein in detergent micelles, which may differ significantly from its native membrane-embedded conformation. Avoid extrapolating results to in vivo structure-function relationships without validation.
2. Antibody Development and Validation
This recombinant STEAP1 is suitable as an immunogen for antibody production, as antibodies can recognize linear epitopes even in misfolded proteins. However, antibodies generated may not recognize the native, properly folded, and membrane-embedded STEAP1 in cellular contexts. Validate antibody specificity against full-length STEAP1 expressed in mammalian cells.
3. Protein-Protein Interaction Studies
Use with extreme caution. While the His-tag enables pull-down assays, the potentially misfolded STEAP1 may exhibit non-physiological interactions. Membrane proteins typically require their native lipid environment for correct interaction interfaces. Any identified partners must be validated using full-length STEAP1 in membrane preparations or cellular systems.
4. Membrane Protein Reconstitution Studies
This is the most appropriate application if successful reconstitution is achieved. The protein can be incorporated into proteoliposomes or nanodiscs to study membrane integration. However, if the initial protein is misfolded from cell-free expression, reconstitution will not restore native structure or function. First, validate folding spectroscopically, and after reconstitution, test metalloreductase activity to confirm functionality.
Final Recommendation & Action Plan
Before using this recombinant STEAP1 for any application, prioritize experimental validation of its structural integrity and potential activity. Start with biophysical characterization (circular dichroism to check secondary structure content against predictions, and analytical ultracentrifugation to assess monodispersity). For functional studies, attempt reconstitution into proteoliposomes with appropriate lipids, then test ferric reductase activity with specific substrates. If activity is confirmed, proceed with interaction or structural studies; otherwise, limit use to antibody production with the caveat that antibodies may require extensive validation against native protein. For reliable results with this complex membrane protein, consider alternative expression systems such as mammalian or insect cells that can provide the proper membrane environment for folding.