Recombinant Human NPC intracellular cholesterol transporter 1 (NPC1) is produced in a yeast expression system, spanning amino acid region 23-261. This partial-length protein comes with a C-terminal 6xHis-Myc tag for easier purification and detection. The product shows greater than 90% purity when confirmed by SDS-PAGE, which appears to provide reliable results in research applications. It's recommended for research use only, though no endotoxin level has been specified.
NPC1 is a critical protein involved in moving cholesterol around inside cells. It seems to play a significant role in keeping cellular lipid balance by helping cholesterol move within lysosomes. The protein's function is essential for understanding cholesterol metabolism and related pathways - this makes NPC1 an important target in lipid research and similar studies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
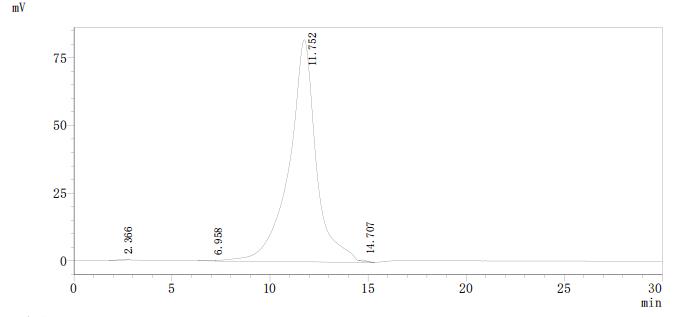
Based on the provided information, the folding state and bioactivity of this recombinant NPC1 protein fragment are unknown and must be considered highly uncertain. NPC1 is a complex multi-pass transmembrane protein with its N-terminal domain (NTD, residues ~25-264) folding into a sterol-binding pocket. While the expressed region (23-261aa) corresponds to this critical NTD, its correct folding is not guaranteed. Expression in yeast (a eukaryotic system) is favorable, but the presence of a C-terminal 6xHis-Myc tag could potentially disrupt the domain's structure or sterol-binding site. The high purity by SEC-HPLC (>95%) suggests a monodisperse preparation, which is a positive indicator of folding homogeneity, but it does not confirm the native, bioactive conformation required for cholesterol binding. Therefore, applications relying on specific biological activity are speculative without validation.
1. Antibody Development and Validation
This recombinant NPC1 fragment is suitable for use as an immunogen to generate antibodies. The high purity and defined region make it a good antigen. However, it is critical to note that the antibodies generated will be against this recombinant NPC1 fragment. Their ability to recognize the natively folded NTD within the full-length, membrane-embedded NPC1 protein in its cellular context (e.g., the lysosomal membrane) is not guaranteed and requires empirical validation. The recombinant NPC1 protein is reliable for developing antibodies for techniques like Western blotting against denatured samples.
2. Protein-Protein Interaction Studies
The dual His-Myc tagging system allows for immobilization in pull-down or co-IP experiments. However, the utility for discovering physiologically relevant binding partners is contingent upon correct folding. The NPC1 NTD's primary known function is cholesterol binding, not protein-protein interaction. Using this unvalidated recombinant NPC1 fragment as bait is high-risk; it may identify non-specific interactors. The suggestion of using it in yeast two-hybrid screens is particularly problematic, as the fragment's folding in the yeast nucleus is unpredictable. This application is not recommended without prior confirmation of native folding.
3. Biochemical Characterization and Functional Domain Mapping
This purified recombinant NPC1 fragment is well-suited for basic biochemical and biophysical characterization of the polypeptide. Studies on its thermal stability, protease sensitivity, and general solution behavior can be performed. True functional mapping (e.g., identifying the cholesterol-binding site) can only be pursued if cholesterol-binding activity is first confirmed. Without activity validation, these studies only characterize the physical properties of the recombinant NPC1, not the functional domain.
4. ELISA-Based Binding Assays
The tagged NPC1 protein can be immobilized for ELISA. However, its use for "screening potential ligands or inhibitors" or testing interactions with "cholesterol derivatives" is entirely dependent on the unverified assumption that the protein is correctly folded and bioactive. If the fragment is misfolded, any binding data will be irrelevant to NPC1's true function. This application should not be attempted without first establishing a functional cholesterol-binding assay to validate the recombinant NPC1 protein's activity. A negative result in such an assay would render ELISA-based screening useless.
Final Recommendation & Action Plan
The immediate and essential first step is to functionally validate this recombinant NPC1 N-terminal domain fragment by performing a cholesterol-binding assay (e.g., using fluorescently labeled cholesterol or a competitive binding assay) to confirm it adopts a bioactive conformation. The high SEC-HPLC purity is a positive starting point. If binding activity is confirmed, the protein becomes highly valuable for Applications 3 (detailed biophysical characterization of the binding domain) and 4 (ligand screening). If inactive, its use should be restricted to Application 1 (antibody production against linear epitopes, with the caveat of limited recognition of the native protein) and the non-functional aspects of Application 3 (basic biophysical characterization of the misfolded fragment). Application 2 (protein interaction studies) is not recommended due to the low probability of the NTD being a major protein interaction hub and the high risk of artifacts with an unvalidated bait protein.