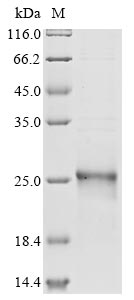
The recombinant human GPX4 protein with an N-terminal 6xHis tag is expressed using an E. coli system. The GPX4 gene fragment [29-197aa (U73S)] is co-cloned into an expression plasmid with the tag gene. The recombinant plasmid is introduced into E. coli cells, which are cultured for protein expression. Protein expression is induced by IPTG, and the cells are lysed to release the recombinant GPX4 protein. Protein purification is achieved using Ni-NTA affinity chromatography, where the His tag binds to nickel ions, enabling the selective purification of GPX4. The eluted GPX4 protein is analyzed by SDS-PAGE, revealing a purity of greater than 90%.
Human GPX4 is a crucial selenoenzyme that plays a significant role in cellular antioxidant defense mechanisms, particularly in the reduction of lipid hydroperoxides and the maintenance of redox homeostasis. Its unique function as the only known enzyme capable of reducing phospholipid hydroperoxides distinguishes it from other glutathione peroxidases, which primarily target hydrogen peroxide and organic hydroperoxides [1][2]. The enzyme's activity is critically dependent on a selenocysteine residue, which is essential for its catalytic function [3].
GPX4 is involved in regulating ferroptosis [1][4]. The enzyme's ability to mitigate oxidative stress is vital for cell survival, particularly in tissues with high oxidative demands, such as the brain and the gastrointestinal tract [5]. GPX4's role extends beyond mere antioxidant activity. It also participates in signaling pathways that affect cell proliferation and apoptosis. In cancer biology, GPX4 has been identified as a potential therapeutic target, as its inhibition can enhance the efficacy of chemotherapeutic agents like cisplatin by promoting ferroptosis cell death in tumor cells [6]. This dual role of GPX4 in both protecting against oxidative damage and facilitating cell death in cancer cells highlights its importance in both health and disease.
References:
[1] W. Yang, R. Sriramaratnam, M. Welsch, K. Shimada, R. Skouta, V. Viswanathan, et al. Regulation of ferroptotic cancer cell death by gpx4, Cell, vol. 156, no. 1-2, p. 317-331, 2014. https://doi.org/10.1016/j.cell.2013.12.010
[2] J. Angeli, M. Schneider, B. Proneth, Y. Tyurina, V. Tyurin, V. Hammond, et al. Inactivation of the ferroptosis regulator gpx4 triggers acute renal failure in mice, Nature Cell Biology, vol. 16, no. 12, p. 1180-1191, 2014. https://doi.org/10.1038/ncb3064
[3] S. Zhu, Q. Zhang, X. Sun, H. Zeh, M. Lotze, R. Kang, et al. Hspa5 regulates ferroptotic cell death in cancer cells, Cancer Research, vol. 77, no. 8, p. 2064-2077, 2017. https://doi.org/10.1158/0008-5472.can-16-1979
[4] M. Gaschler, A. Andia, H. Liu, J. Csuka, B. Hurlocker, C. Vaianaet al., Fino2 initiates ferroptosis through gpx4 inactivation and iron oxidation, Nature Chemical Biology, vol. 14, no. 5, p. 507-515, 2018. https://doi.org/10.1038/s41589-018-0031-6
[5] B. Speckmann, H. Bidmon, A. Pinto, M. Anlauf, H. Sies, & H. Steinbrenner, Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation, Journal of Biological Chemistry, vol. 286, no. 12, p. 10764-10772, 2011. https://doi.org/10.1074/jbc.m110.216028
[6] X. Zhang, S. Sui, L. Wang, H. Li, L. Zhang, S. Xu, et al. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin, Journal of Cellular Physiology, vol. 235, no. 4, p. 3425-3437, 2019. https://doi.org/10.1002/jcp.29232