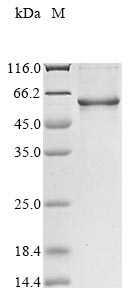
The recombinant human VTN protein is produced by inserting the VTN gene fragment (20-478aa) into a plasmid vector, followed by introducing the recombinant vectors into E.coli cells. Once expressed, the VTN protein is subjected to multiple purification processes involving affinity chromatography. Following purification, SDS-PAGE is employed to check the protein's purity, exceeding 85%.
Human vitronectin (VTN) is a multifunctional glycoprotein that plays a crucial role in various biological processes, including cell adhesion, migration, and the regulation of the immune response. It is predominantly synthesized in the liver and is found in the extracellular matrix (ECM) as well as in plasma [1][2]. Vitronectin is primarily involved in cell adhesion through its interaction with integrins, particularly the αvβ3 and αvβ5 integrins, via its Arg-Gly-Asp (RGD) domain [3][4][5]. This adhesive property is critical for various physiological processes, including wound healing, tissue repair, and the metastatic spread of cancer cells [6][7]. In the context of cancer, altered vitronectin levels have been associated with different stages of tumor progression, suggesting its potential as a biomarker for certain malignancies, including breast and ovarian cancers [6][7][8].
Moreover, vitronectin is implicated in regulating the complement system, particularly in inhibiting the formation of the membrane attack complex (MAC), which is essential for protecting host cells from lysis by the immune system [9]. This function is particularly relevant in bacterial infections, where pathogens such as Neisseria meningitidis and Yersinia pestis exploit vitronectin to enhance their adhesion to host cells and evade immune responses [1][5].
References:
[1] C. Cunha, N. Griffiths, & M. Virji. Neisseria meningitidis opc invasin binds to the sulphated tyrosines of activated vitronectin to attach to and invade human brain endothelial cells, Plos Pathogens, vol. 6, no. 5, p. e1000911, 2010. https://doi.org/10.1371/journal.ppat.1000911
[2] C. Park, M. Song, S. Kim, & B. Min. Vitronectin-derived peptide promotes reparative dentin formation, Journal of Dental Research, vol. 101, no. 12, p. 1481-1489, 2022. https://doi.org/10.1177/00220345221101506
[3] A. Zhou. Functional structure of the somatomedin b domain of vitronectin, Protein Science, vol. 16, no. 7, p. 1502-1508, 2007. https://doi.org/10.1110/ps.072819107
[4] A. Attia, S. Ram, P. Rice, & E. Hansen. Binding of vitronectin by themoraxella catarrhalisuspa2 protein interferes with late stages of the complement cascade, Infection and Immunity, vol. 74, no. 3, p. 1597-1611, 2006. https://doi.org/10.1128/iai.74.3.1597-1611.2006
[5] S. Bartra, Y. Ding, .et al. Yersinia pestis uses the ail outer membrane protein to recruit vitronectin, Microbiology, vol. 161, no. 11, p. 2174-2183, 2015. https://doi.org/10.1099/mic.0.000179
[6] A. Bera, M. Subramanian, et al. Functional role of vitronectin in breast cancer, Plos One, vol. 15, no. 11, p. e0242141, 2020. https://doi.org/10.1371/journal.pone.0242141
[7] G. Schneider, M. Suszynska, S. Kakar, & M. Ratajczak. Vitronectin in the ascites of human ovarian carcinoma acts as a potent chemoattractant for ovarian carcinoma: implication for metastasis by cancer stem cells, Journal of Cancer Stem Cell Research, vol. 4, no. 5, p. 1, 2016. https://doi.org/10.14343/jcscr.2016.4e1005
[8] J. Leroy-Dudal, L. Heyman, P. Gauduchon, & F. Carreiras. Adhesion of human ovarian adenocarcinoma igrov1 cells to endothelial cells is partly mediated by the αv integrins–vitronectin adhesive system and induces an alteration of endothelial integrity, Cell Biology International, vol. 29, no. 6, p. 482-488, 2005. https://doi.org/10.1016/j.cellbi.2005.01.008
[9] L. Carreras-Planella, D. Cucchiari, et al. Urinary vitronectin identifies patients with high levels of fibrosis in kidney grafts, Journal of Nephrology, vol. 34, no. 3, p. 861-874, 2020. https://doi.org/10.1007/s40620-020-00886-y