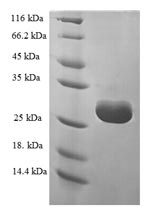
Amino acids 1-125 constitute the expression domain of recombinant Human TRMT112. The expected molecular weight for the TRMT112 protein is calculated to be 30.2 kDa. Expression of this TRMT112 protein is conducted in e.coli. The N-terminal 6xHis-SUMO tag was fused into the coding gene segment of TRMT112, making it easier to detect and purify the TRMT112 recombinant protein in the later stages of expression and purification.
The human tRNA methyltransferase 112 homolog (TRMT112) is a protein involved in tRNA modification. TRMT112 is essential for the proper functioning of the tRNA modification machinery. TRMT112 associates with various tRNA methyltransferases, aiding in the methylation of specific nucleotides within tRNAs. This methylation process contributes to the structural stability and accurate translation of mRNA into proteins during protein synthesis. Research on TRMT112 extends to understanding its role in ensuring the fidelity of translation and its potential implications in various cellular processes. Exploring TRMT112 provides valuable insights into the intricate regulatory mechanisms governing protein synthesis and broader cellular functions.