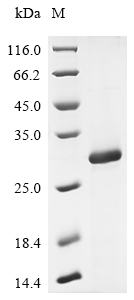
Recombinant Macaca fascicularis Interleukin-2 receptor subunit beta (IL2RB) is expressed in E. coli and contains amino acids 27 to 240 of the protein, representing a partial sequence. An N-terminal 6xHis-tag is included to simplify purification and detection processes. SDS-PAGE analysis confirms the protein achieves greater than 85% purity, which appears suitable for most research applications.
Interleukin-2 receptor subunit beta (IL2RB) serves as a key component within the interleukin-2 receptor complex. Its role in immune response regulation seems particularly important. The protein participates in signal transduction pathways that may control T cell growth, differentiation, and survival. Researchers studying immune function and therapeutic development will likely find this protein valuable for their work.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The E. coli system lacks eukaryotic chaperones and post-translational modification machinery, increasing the risk of misfolding or aggregation, particularly for a complex protein like IL2RB, which requires precise disulfide bond formation and tertiary structure for functional activity (e.g., IL-2 binding). While the partial sequence includes key functional domains, the N-terminal His-tag could sterically interfere with folding or binding interfaces. Without validation (e.g., circular dichroism for secondary structure, size-exclusion chromatography for oligomeric state, or IL-2 binding assays), the protein cannot be assumed to be correctly folded or bioactive.
1. Protein-Protein Interaction Studies
This recombinant IL2RB extracellular domain could be used to study interactions with IL-2 or other receptor subunits only if its folding is experimentally verified. The His-tag enables technical immobilization for SPR or BLI assays. However, if the protein is misfolded, its binding interfaces may be altered, leading to non-physiological interactions (e.g., false positives/negatives in IL-2 binding kinetics). The E. coli expression system may not support native disulfide bonding or conformational epitopes, compromising results. Any data requires validation with full-length, native IL2RB from mammalian cells or primate tissues.
2. Antibody Development and Characterization
The recombinant IL2RB protein is suitable as an immunogen for generating antibodies targeting linear epitopes, as antibody production often relies on amino acid sequences rather than native conformation. The high purity (>85%) reduces contamination risks. However, if the protein is misfolded, antibodies may not recognize the native IL2RB in physiological contexts (e.g., on cell surfaces), particularly if conformational epitopes are critical. The His-tag could also induce tag-specific antibodies, requiring careful screening for specificity.
3. Structural and Biochemical Analysis
The protein's suitability for structural studies (e.g., X-ray crystallography) is highly dependent on correct folding and homogeneity. Techniques like circular dichroism could assess secondary structure, but data from a misfolded protein would not reflect the native IL2RB architecture. The partial sequence (lacking transmembrane and intracellular domains) and His-tag might disrupt oligomerization or crystal packing. Prior validation of folding and monodispersity (e.g., via SEC-MALS) is essential.
4. Comparative Species Analysis
This application could provide evolutionary insights if the protein is correctly folded. However, misfolding could lead to erroneous conclusions about species-specific differences (e.g., falsely attributing reduced IL-2 binding to sequence divergence rather than poor folding). Comparisons with human or other primate IL2RB versions should be cross-validated using proteins expressed in mammalian systems or cell-based assays to ensure biological relevance.
Final Recommendation & Action Plan
To ensure reliable outcomes, prioritize experimental validation of the protein's folding and bioactivity before any functional application. Start with biophysical characterization (e.g., size-exclusion chromatography with multi-angle light scattering to assess oligomeric state and circular dichroism spectroscopy to evaluate secondary structure). Then, perform functional assays such as an IL-2 binding test using surface plasmon resonance or ELISA to confirm bioactivity. If validation succeeds, the protein can be cautiously used for the proposed applications, with disclosures about tag and partial-sequence limitations. If validation fails, restrict use to non-conformation-dependent applications like linear-epitope antibody production, and always state limitations in research communications.