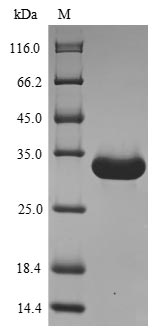
Recombinant Mouse Angiogenin-4 (Ang4) is produced in a yeast expression system and contains the full-length mature protein sequence, specifically amino acids 25-144. An N-terminal 6xHis-sumostar tag is included to make purification and detection more straightforward. SDS-PAGE analysis shows the product has greater than 90% purity, which appears to make it suitable for various research applications that need high-quality protein samples.
Angiogenin-4 (Ang4) is a protein that's involved in angiogenesis - the process where new blood vessels form. This process seems crucial in many normal body functions as well as disease states. Ang4 belongs to the ribonuclease A superfamily and likely plays a role in how cells respond to changes in their surrounding environment. Researchers are particularly interested in Ang4 when studying blood vessel development and the signaling pathways that control it.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Based on the provided information, the recombinant mouse Ang4 protein is unlikely to be correctly folded or fully bioactive without experimental validation. Ang4 is a member of the RNase A superfamily and requires precise folding, including disulfide bond formation, for its ribonuclease activity. The yeast expression system may support eukaryotic-like folding and disulfide bonding, but the N-terminal 6xHis-SUMOstar tag is large (approximately 15-20 kDa) compared to the mature Ang4 domain (∼13 kDa, 25-144aa), which could sterically hinder proper folding, particularly near the active site. The tag may disrupt the tertiary structure essential for enzymatic function. Without validation (e.g., circular dichroism for secondary structure, ribonuclease activity assays), the protein's folding and bioactivity remain uncertain. Therefore, while the eukaryotic system offers some advantages, the large tag poses a high risk of misfolding.
This recombinant Ang4 can generate antibodies, but they will primarily target the immunodominant SUMOstar tag rather than Ang4-specific epitopes. If correctly folded, antibodies might recognize native conformations; if misfolded, they may detect non-physiological epitopes, reducing utility for detecting endogenous Ang4. For specific antibodies, remove the tag before immunization or use tag-free protein for validation assays. The high purity minimizes cross-reactivity with contaminants but not with the tag itself.
Given the substantial interference from the large SUMOstar tag, it is critical to first remove the tag using SUMO protease (if the construct includes a cleavage site) and purify the tag-free Ang4 domain. Then, validate folding and bioactivity using techniques such as circular dichroism to confirm expected secondary structure (e.g., alpha-helical content typical of RNase A family), ribonuclease activity assays with synthetic RNA substrates to measure enzymatic function, and size-exclusion chromatography to assess oligomeric state. If tag removal is not feasible, limit applications to tag-centric uses (e.g., anti-SUMOstar antibody development) and avoid functional studies like enzymatic assays or interaction mapping. For all applications, include controls such as tag-free Ang4 or known bioactive standards to account for potential artifacts.