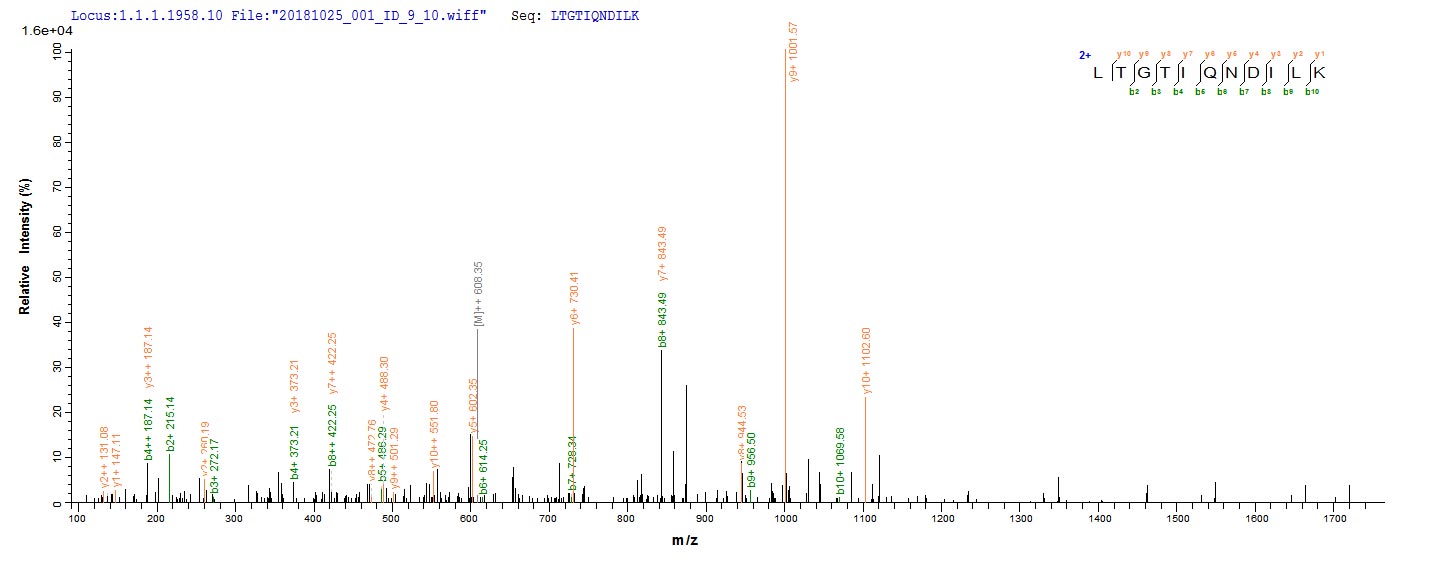
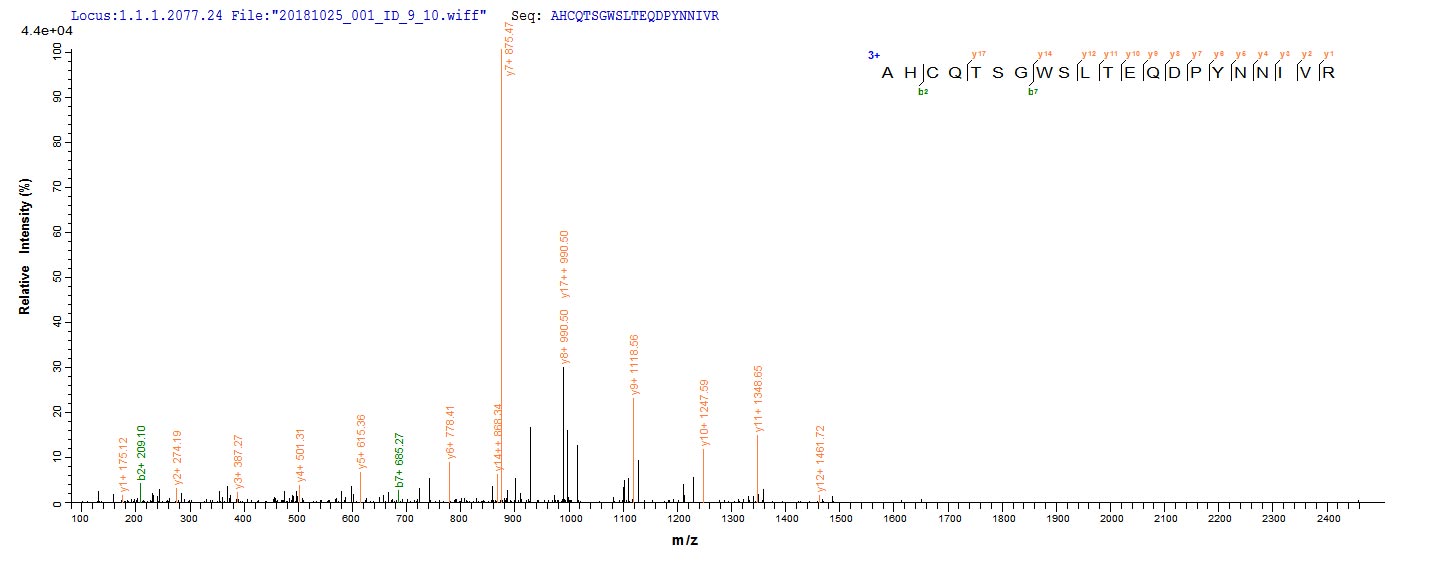
Recombinant Mouse Methylmalonyl-CoA mutase, mitochondrial (Mutc) is expressed in E.coli and covers the full length of the mature protein from amino acids 31 to 748. The protein carries an N-terminal 6xHis tag, which makes purification and detection more straightforward. Purity exceeds 90% according to SDS-PAGE verification, though this should provide reliable results for most experimental applications. This product is intended for research use only.
Methylmalonyl-CoA mutase plays a crucial role in cellular metabolism—specifically converting methylmalonyl-CoA to succinyl-CoA. This conversion appears vital for breaking down certain amino acids and fatty acids. The enzyme's activity seems essential for metabolic pathways that keep normal cellular function intact. Given this central role, Mutc has become a significant focus in metabolic research. Scientists are using it to better understand energy production and various metabolic disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Methylmalonyl-CoA mutase is a complex mitochondrial enzyme that requires adenosylcobalamin (vitamin B12) as a cofactor and forms homodimers for functionality. The E. coli expression system can produce the protein, but cannot incorporate the essential B12 cofactor or ensure a proper mitochondrial folding environment. While the protein may achieve some structural elements, it will lack the cofactor necessary for enzymatic activity. The probability of correct functional folding is moderate, but the protein will be inactive without B12 supplementation.
1. Protein-Protein Interaction Studies Using His-Tag Pull-Down Assays
This application carries a moderate risk. If correctly folded, the protein could identify physiological interaction partners. However, without the B12 cofactor, the protein may be misfolded or unstable, leading to non-specific binding or failure to recognize genuine partners. The His-tag may also cause steric interference with interaction surfaces. Protein-protein interactions require proper folding, but the lack of cofactor incorporation increases the risk of aberrant results. Validation with endogenous protein is essential.
2. Antibody Development and Validation
This recombinant Mutc serves as an excellent immunogen for generating antibodies against mouse methylmalonyl-CoA mutase. The full-length mature sequence ensures comprehensive epitope coverage. The high purity minimizes antibodies against contaminants. Resulting antibodies will be valuable for detecting both apo- and holo-forms of the enzyme.
3. Biochemical Characterization and Cofactor Binding Studies
This is the critical first step to assess protein quality and function. Techniques like size-exclusion chromatography can determine oligomeric state, while UV-visible spectroscopy can detect B12 incorporation after in vitro reconstitution. Activity assays with supplemented cofactor can validate enzymatic function. These studies directly address the folding and activity uncertainties, providing essential data for evaluating functional capability.
4. Comparative Species Analysis
This protein can be used for structural and sequence comparisons, but not for functional comparative studies. Sequence alignment, immunochemical cross-reactivity, and biophysical property comparisons are feasible. However, enzymatic activity comparisons across species require all proteins to be fully active with incorporated cofactors.
Final Recommendation & Action Plan
This recombinant Mutc has potential for functional applications but requires cofactor incorporation and folding validation before reliable use in interaction or comparative functional studies. The immediate priority is Application 3 (Biochemical Characterization) to assess protein folding, oligomeric state, and most importantly, to test B12 incorporation and enzymatic activity after in vitro reconstitution. If the protein demonstrates proper cofactor binding and activity, it becomes suitable for Applications 1 and 4 (functional comparisons). Application 2 (Antibody Development) can proceed immediately regardless of functional status. For all functional applications, B12 supplementation is essential, and results should be validated with positive controls. This systematic approach ensures reliable interpretation of experimental outcomes.