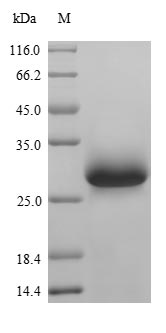
Recombinant Mouse von Willebrand factor (Vwf) is produced through a yeast expression system and covers amino acids 1498 to 1665 of the protein. This partial protein comes with an N-terminal 6xHis-tag and a C-terminal Myc-tag, which makes purification and detection much more straightforward. The product shows purity greater than 85% when verified by SDS-PAGE. This level appears sufficient for reliable research applications across different experimental settings.
Von Willebrand factor represents a crucial protein in hemostasis, working as a mediator in platelet adhesion and aggregation processes after vascular injury occurs. It's an essential component of the blood coagulation pathway, helping platelets bind to damaged endothelium and stabilizing blood clot formation. Research on von Willebrand factor may be vital for understanding bleeding disorders and developing new therapeutic strategies.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
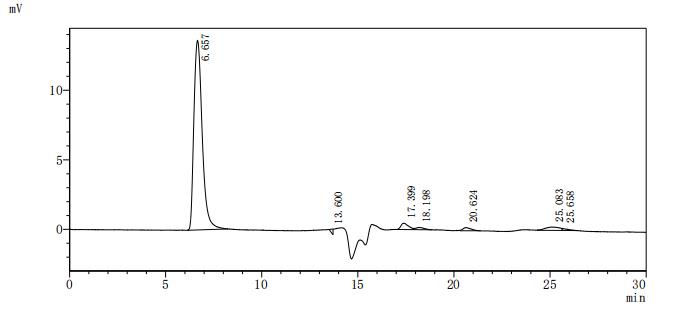
Based on the provided information, the folding state and bioactivity of this recombinant mouse Vwf protein fragment are unknown and must be considered highly uncertain. VWF is a large, complex multimeric glycoprotein whose function critically depends on extensive disulfide bond formation, specific glycosylation patterns, and higher-order oligomerization. Expressing a small fragment (1498-1665aa) corresponding to a small portion of the mature subunit in a yeast system, while eukaryotic, is unlikely to replicate the complex post-translational processing and correct disulfide bonding of the native protein. The presence of tags on both termini further complicates correct folding. The high purity by SEC-HPLC (>90%) suggests a monodisperse preparation but does not confirm a natively folded, bioactive structure capable of binding its ligands (e.g., collagen, platelets via GPIb). Therefore, applications relying on specific biological interactions are speculative.
1. Antibody Development and Validation Studies
This recombinant Vwf fragment is suitable for use as an immunogen to generate antibodies specific to this region of mouse Vwf. The dual tags facilitate purification and screening. However, it is critical to understand that the antibodies generated will be against the linear epitopes of this non-native fragment. Their ability to recognize the full-length, correctly folded, and highly multimeric native VWF in mouse plasma or tissues is very low and must be rigorously validated. The purity level is adequate for immunization, but the primary utility of these antibodies will be for detecting denatured VWF (Western blot) or this specific immunogen.
2. Comparative Species Analysis and Cross-Reactivity Studies
This application is valid and well-suited for this protein. The recombinant VWF fragment can be used effectively to map species-specific linear epitopes and test the cross-reactivity of antibodies raised against human or other species' VWF. This is a valuable application for characterizing antibody specificity, as it relies on sequence comparison rather than native folding.
3. ELISA Development and Immunoassay Standardization
The dual-tagged recombinant VWF protein can be used to develop an ELISA. However, its utility is severely limited. It is suitable for developing assays to detect antibodies raised against this specific immunogen (Application 1). It is not suitable as a standard for "mouse Vwf detection and quantification" in biological samples because it is antigenically distinct from the native, full-length, multimeric VWF in plasma. An assay using this reagent would not accurately measure endogenous VWF levels.
Final Recommendation & Action Plan
The recommended course of action is to restrict the use of this recombinat VWF protein lot to Applications 1 (antibody development with the explicit goal of generating tools for linear epitopes) and 2 (comparative species analysis), which are independent of native folding. Interaction studies and the quantitative aspects of Application 3 (ELISA for native protein quantification) must be abandoned, as they will yield biologically irrelevant data. For any functional studies related to VWF (ligand binding, multimer analysis), a full-length or much larger functional domain (e.g., the A1 domain for GPIb binding) must be produced in a mammalian expression system capable of supporting the necessary complex glycosylation and disulfide bond formation.