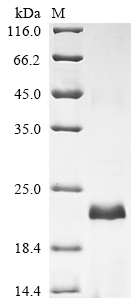
The process of producing recombinant rat Pla2g10 in E. coli involves co-inserting the target gene into an expression vector with an N-terminal 10xHis-tag and C-terminal Myc-tag gene, which is introduced into E. coli cells. The target gene codes for the 29-151aa of rat Pla2g10 protein. The cells are grown and induced to express the rat Pla2g10 protein. The cells are lysed to release the expressed Pla2g10 protein, which is purified using affinity chromatography technique. Protein purity is assessed using SDS-PAGE, exceeding 90%.
PLA2G10 protein is a phospholipase A2 enzyme in various physiological processes. PLA2G10 has been implicated in cancer progression, where its catalytic activity contributes to impairing T-cell infiltration, thereby dampening immunity [1]. It modulates lipid metabolism, promoting breast cancer cell growth and survival by stimulating lipid droplet formation and fatty acid oxidation [2]. In the context of colon tumorigenesis, PLA2G10 exhibits potent activity in releasing arachidonic acid, leading to prostaglandin E2 formation [3].
Furthermore, PLA2G10 has been associated with various physiological functions beyond cancer. For example, it has been identified as a novel progesterone receptor target gene induced in the uterine luminal epithelium, playing a role in uterine receptivity for embryo implantation [4]. Additionally, PLA2G10 has been linked to skin homeostasis, with its enzymatic activity transiently elevated before hair loss, suggesting a role in hair follicular function [5]. Moreover, studies have shown that PLA2G10 deficiency can lead to reduced myocardial ischemia-reperfusion injury, indicating its involvement in inflammatory responses and tissue damage [6].
References:
[1] T. Zhang, Up-regulated pla2g10 in cancer impairs t cell infiltration to dampen immunity, Science Immunology, vol. 9, no. 94, 2024. https://doi.org/10.1126/sciimmunol.adh2334
[2] S. Park, E. Huang, & T. Ahn, Classification and functional analysis between cancer and normal tissues using explainable pathway deep learning through rna-sequencing gene expression, International Journal of Molecular Sciences, vol. 22, no. 21, p. 11531, 2021. https://doi.org/10.3390/ijms222111531
[3] Y. Liu, X. Zhu, J. Zhu, S. Liao, Q. Tang, K. Liuet al., Identification of differential expression of genes in hepatocellular carcinoma by suppression subtractive hybridization combined cdna microarray, Oncology Reports, 2007. https://doi.org/10.3892/or.18.4.943
[4] H. Park, S. Park, M. Lee, G. Kim, S. Park, S. Yanget al., Secretory phospholipase a2-x (pla2g10) is a novel progesterone receptor target gene exclusively induced in uterine luminal epithelium for uterine receptivity in mice,, 2020. https://doi.org/10.21203/rs.3.rs-41891/v2
[5] K. Yamamoto, Y. Taketomi, Y. Isogai, Y. Miki, H. Sato, S. Masudaet al., Hair follicular expression and function of group x secreted phospholipase a2 in mouse skin, Journal of Biological Chemistry, vol. 286, no. 13, p. 11616-11631, 2011. https://doi.org/10.1074/jbc.m110.206714
[6] S. Gora, C. Perret, I. Jemel, V. Nicaud, G. Lambeau, F. Cambienet al., Molecular and functional characterization of polymorphisms in the secreted phospholipase a2 group x gene: relevance to coronary artery disease, Journal of Molecular Medicine, vol. 87, no. 7, p. 723-733, 2009. https://doi.org/10.1007/s00109-009-0483-y
[7] Q. Huang, Y. Wu, C. Qin, W. He, & X. Wei, Phylogenetic and structural analysis of the phospholipase a2 gene family in vertebrates, International Journal of Molecular Medicine, vol. 35, no. 3, p. 587-596, 2014. https://doi.org/10.3892/ijmm.2014.2047