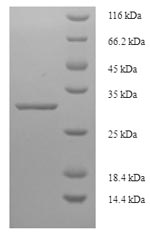
Recombinant Triticum monococcum Omega-gliadin is produced in E. coli and comes with an N-terminal GST tag, which makes purification and detection more straightforward. This protein represents a partial-length sequence covering the 1-28 amino acid region and reaches over 90% purity according to SDS-PAGE analysis. Such high purity levels appear to provide reliable performance for various research applications, particularly when endotoxin levels aren't a major concern.
Omega-gliadin from Triticum monococcum—a type of einkorn wheat—functions as a seed storage protein that primarily handles the nutritional storage of amino acids. It seems to play an important role in plant development and has drawn interest from researchers working on cereal crop improvement and gluten-related studies. Understanding its structure and function may contribute to advances in agricultural biotechnology and nutritional science.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Triticum monococcum Omega-gliadin is a gluten protein that requires precise folding and specific tertiary structure for its functional activity in gluten network formation and immunogenicity. The E. coli expression system may not support optimal folding for eukaryotic proteins, but the small fragment size (28 aa) simplifies folding. However, the large N-terminal GST tag (∼26 kDa) is significantly larger than the peptide itself (∼3 kDa), causing severe steric interference and dominating the protein's properties. The probability of correct folding with functional bioactivity is extremely low, as the tag may mask functional domains and prevent native interactions.
1. Antibody Development and Epitope Mapping
This application has severe limitations. While antibodies can be generated, the immune response will primarily target the large foreign GST tag rather than the small gliadin fragment. Antibodies may not recognize native, full-length omega-gliadin in its physiological context, limiting utility for immunological studies.
2. GST-Tagged Protein Purification Method Development
This application is highly suitable for technical optimization. The GST tag allows for efficient purification via glutathione affinity chromatography, and the high purity (>90%) makes it a good model for developing and refining protein purification protocols in E. coli systems.
Final Recommendation & Action Plan
This GST-tagged omega-gliadin fragment is unsuitable for functional studies due to the extreme size disparity between the tag and peptide, which causes severe steric interference. Avoid interaction studies and functional studies entirely, as they would yield biologically meaningless results. Application 2 (antibody development) has severe limitations and is unlikely to produce antibodies useful for native protein recognition. Only Application 2 (purification method development) is highly suitable for technical optimization. For reliable omega-gliadin research, use full-length or larger tag-free fragments expressed in eukaryotic systems to preserve native structure and function.