Alternative Names
Galactose-1-phosphate uridylyltransferase (Gal-1-P uridylyltransferase) (EC 2.7.7.12) (UDP-glucose--hexose-1-phosphate uridylyltransferase), GALT
Immunogen
A synthesized peptide derived from human GALT
Immunogen Species
Homo sapiens (Human)
Purification Method
Affinity-chromatography
Concentration
It differs from different batches. Please contact us to confirm it.
Buffer
Rabbit IgG in 10mM phosphate buffered saline , pH 7.4, 150mM sodium chloride, 0.05% BSA, 0.02% sodium azide and 50% glycerol.
Tested Applications
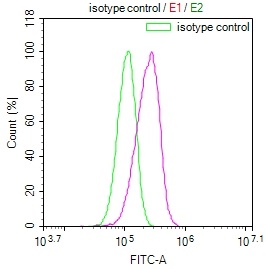
ELISA, FC
Recommended Dilution
| Application |
Recommended Dilution |
| FC |
1:50-1:200 |
Storage
Upon receipt, store at -20°C or -80°C. Avoid repeated freeze.
Lead Time
Basically, we can dispatch the products out in 1-3 working days after receiving your orders. Delivery time maybe differs from different purchasing way or location, please kindly consult your local distributors for specific delivery time.
Description
The GALT recombinant monoclonal antibody was meticulously generated by CUSABIO through a rigorous procedure. Initially, B cells were isolated from the spleen of an immunized animal, with a synthesized peptide derived from human GALT used as the immunogen. Subsequently, RNA was extracted from the B cells and converted into cDNA through reverse transcription. Using the cDNA as a template, the gene encoding the GALT antibody was amplified with a degenerate primer and inserted into a vector. The recombinant vector was then introduced into host cells through transfection, enabling the production of the GALT recombinant monoclonal antibodies. Following expression, these antibodies were purified from the cell culture supernatant using affinity chromatography. The GALT recombinant monoclonal antibody's specific reactivity with human GALT protein was confirmed through ELISA and FC applications.
Usage
For Research Use Only. Not for use in diagnostic or therapeutic procedures.