2. Related Signaling Pathways in Breast Cancer
General speaking, signal transduction is critical in the development and treatment of cancer. Together with recent research, we have summarized the signaling pathways associated with breast cancer.
NF-κB Signaling Pathway and Breast Cancer
Nuclear factor kappa-light-chain-enhancer of activated B cells, also known as nuclear factor-kappa B (NF-kB), is a heterodimeric DNA-binding protein that consists of two major subunits, p50 and p65. NF-κB is widely used by eukaryotic cells as a regulator of genes that control cell proliferation and cell survival. In cancer, the crucial regulator proteins of NF-κB signaling pathway are mutated or aberrantly expressed, leading to defective coordination between the malignant cell and the rest of the organism. This is evident both in metastasis, as well as in the inefficient eradication of the tumor by the immune system[1]. Activated NF-kB can bind to DNA and lead to the expression of diverse genes that promote cell proliferation, regulate apoptosis, facilitate angiogenesis and stimulate invasion and metastasis[2][3][4]. Accumulating evidence show that NF-κB activity is commonly elevated in breast cancer. For instance, H Nakshatri, et al. reported that NF-kappaB was constitutively active in ER-negative breast cancer cell lines in 1997[5]. Furthermore, NF-κB recently becomes a key target to breast cance treatment. Jisheng Xiao, et al. demonstrated that blocking the NF-kB signaling pathway can inhibit metastasis and growth of breast cancer via using bioreducible PEI-based/p65 shRNA complex nanoparticles[6]. Besides that, several studies also suggest that the NF-κB signaling pathway can become a treatment target of breast cancer[7][8].
TGF-beta Signaling Pathway and Breast Cancer
The transforming growth factor beta (TGFβ) signaling pathway, also known as TGFβ signaling pathway, is involved in many cellular processes including cell growth, cell differentiation, apoptosis, cellular homeostasis and other cellular functions. The TGF-β ligands have three described isoforms; TGF-β1, TGF-β2, and TGF-β3. TGF-β plays a dual role in cancer development as it displays both tumorigenic and tumor-suppressive effects. TGF-β has been reported to act as a tumor suppressor by inhibiting the cell proliferation of breast cancer cell lines[9]. In the research of E. M. de Kruijf, et al., they have demonstrated that the combination of TGF-β pathway biomarkers can provide valuable prognostic value for breast cancer patients. Stratifying tumors according to the low or high expression of TGF-β biomarkers had strong prognostic implications in our patient population. Additionally, their results highlight the importance of accounting for protein expression levels and the complex interactions taking place between components with the TGF-β pathway[10].
PI3/AKT/mTOR Signaling Pathway and Breast Cancer
The PI3K/AKT/mTOR pathway is an intracellular signaling pathway important in regulating the cell cycle and is directly related to cellular quiescence, proliferation, cancer, and longevity. PI3K activation phosphorylates and activates AKT, localizing it in the plasma membrane[11]. The signaling pathway is activated by stimulation of receptor tyrosine kinases, which in turn trigger PI3K activation, followed by phosphorylation of AKT and mTOR complex 1 (mTORC1). In various subtypes of breast cancer, aberrations in the PI3K/AKT/mTOR pathway are the most common genomic abnormalities, including the PIK3CA gene mutation, the loss-of-function mutations or epigenetic silencing of phosphatase, tensin homologue (PTEN), et al[12][13]. Triple-negative breast cancer (TNBC) is defned by the absence of targetable aberrations, such as hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2). In TNBC, oncogenic activation of the PI3K/AKT/mTOR pathway can happen as a function of overexpression of upstream regulators (e.g., epidermal growth factor receptor [EGFR]), activating mutations of PI3K catalytic subunit α (PIK3CA), loss of function or expression of phosphatase and tensin homolog (PTEN), and the proline-rich inositol polyphosphatase, which are downregulators of PI3K[14][15][16]. Recently, emerging preclinical data support the notion that aberrations in the PI3K/AKT/mTOR pathway predict TNBC inhibition by targeted agents[17]. If you want to get more information about the treatment target of PI3K/AKT/mTOR pathway in breast cancer, please click here to view the related article. If you can't see the full text, please contact us via the live chat in the page of lower right.
Notch Signaling Pathway and Breast Cancer
The Notch signaling pathway is a highly conserved, intercellular signaling mechanism essential for proper embryonic development in all metazoan organisms. Mammals possess four different notch receptors (NOTCH1, NOTCH2, NOTCH3, and NOTCH4). Accumulating studies in primary human breast cancer have shown that high-level expression of Jag1 (Jag1High) or Notch1 (Notch1High) mRNA in tumors correlates with poor outcome and is an independent prognostic indicator[18][19]. Emerging evidence has demonstrated that the oncogenic role of Notch in breast cancer is mediated in part via its crosstalk with other signaling pathways, such as the estrogen pathway. Approximately 80% of breast cancers express the estrogen receptor and are treated with anti-estrogens, but resistance to anti-estrogens often develops. One mechanism of resistance may be via the Notch pathway[20]. From a therapeutic standpoint, concurrently targeting both the estrogen receptor and the notch pathway may help to overcome or at least in part delay this resistance.
Several Studies of Other Signaling Pathway in Breast Cancer
In 2011, Mamiko Shimizu, et al. reported that Notch knockdown reduced transcription of uPA and phenocopied uPA knockdown in breast cancer cells. Moreover, the data of their research suggested that JAG1-induced Notch activation results in breast cancer progression by upregulation of the plasminogen activator system, directly linking these 2 important pathways of poor prognosis. Please click here to to view the related article. If you can't see the full text, please contact us via the live chat in the page of lower right.
In addition, Lauren L.C. Marotta, et al. found that the IL-6/JAK2/Stat3 pathway was preferentially active in CD44+CD24- breast cancer cells compared with other tumor cell types, and inhibition of JAK2 decreased their number and blocked growth of xenografts. Furthermore, their results highlight the differences between distinct breast cancer cell types and identify targets such as JAK2 and Stat3 that may lead to more specific and effective breast cancer therapies. Please click here to view the reference. to view the related article. If you can't see the full text, please contact us via the live chat in the page of lower right.
Furthermore, Perrin F. Windham from University of Montevallo found that the cGMP signaling pathway may be aberrantly regulated in breast cancer and can be a target for the prevention and treatment of breast cancer. Please click here to view the reference. to view the related article. If you can't see the full text, please contact us via the live chat in the page of lower right.
This year, Chien-Wei Tseng, from China Medical University, demonstrated that transketolase regulates the metabolic switch to control breast cancer cell metastasis via the alpha-ketoglutarate signaling pathway and can be exploited as a modality for improving therapy. Please click here to view the reference. to view the related article. If you can't see the full text, please contact us via the live chat in the page of lower right.
3. The Most Popular Targets in Breast Cancer Treatment
As you known, breast cancer is the most common female cancer in the world, the second leading cause of cancer death after lung cancer, and the main cause of death in women aged 20 to 59. With the further study of breast cancer mechanism, the target of breast cancer treatment has been gradually found.
HER2 for The Treatment of Breast Cancer
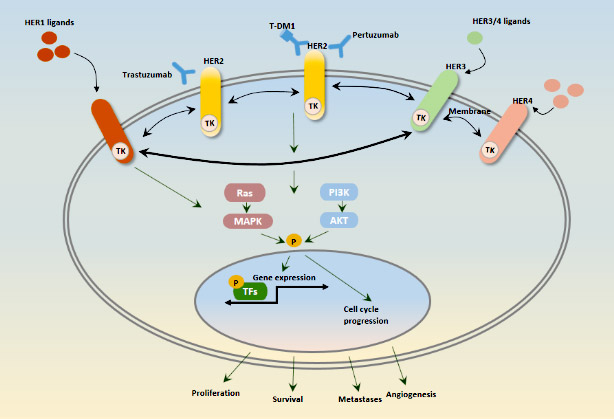
HER2 (also known as ERBB2), a member of the HER family of tyrosine kinase receptors (HER1–4), is an essential breast cancer oncogene. Emerging evidence founded that a significant correlation exists between ERBB2 overexpression and reduced survival of breast cancer patients, and amplification of ERBB2 occurs in 30% of early-stage breast cancers[21]. Treatment with the anti-HER2 monoclonal antibody trastuzumab has revolutionized the outcome of patients with this aggressive breast cancer subtype, but intrinsic and acquired resistance is common. With the further study of breast cancer mechanism, growing understanding of the biology and complexity of the HER2 signaling network and of potential resistance mechanisms has guided the development of new HER2-targeted agents. Combinations of these drugs to more completely inhibit the HER receptor layer, or combining HER2-targeted agents with agents that target downstream signaling, alternative pathways, or components of the host immune system, are being vigorously investigated in the preclinical and clinical settings[22]. As shown in the figure 1, the HER2 network and HER2-targeted therapy for HER2+ breast cancer.
Androgen Receptor for The Treatment of Breast Cancer
Androgen receptor (AR) is a steroid hormonal receptor that belongs to the nuclear receptors family together with estrogen (ER), progesterone (PR), glucocorticoid and mineralcorticoid receptor. Recent data suggest that triple negative breast cancer (TNBC) is not a single disease, but it is rather an umbrella for different ontology-profiles such as mesenchymal, basal like 1 and 2, and the luminal androgen receptor (LAR). The LAR subtype is characterized by the expression of the Androgen Receptor (AR) and its downstream effects. Notwithstanding the role of the AR in several signaling pathways, its impact on a biological and clinical standpoint is still controversial. The LAR subtype has been associated with better prognosis, less chemotherapy responsiveness and lower pathologic complete response after neoadjuvant treatment. Clinical evidence suggests a role for anti-androgen therapies such as bicalutamide, enzalutamide and abiraterone, offering an interesting chemo-free alternative for chemo-unresponsive patients, and therefore potentially shifting current treatment strategies[23].
Beside the two targets, there are still several targets for treatment of breast cancer, such as The CXCL12-CXCR4 chemotactic pathway, Adipose tissue, EP4, et al. These targets not only are genes, but also are tissue or signaling pathway. Despite our remarkable success in breast cancer research, there is still a long way to go to study the mechanism of breast cancer.
References
[1] Vlahopoulos, SA. Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode [J]. Cancer biology & medicine. 2017, 14: 254-270.
[2] Wu Y, Deng J, et al. Stabilization of snail by NF-kB is required for inflammation-induced cell migration and invasion [J]. Cancer Cell. 2009, 15:416-28.>
[3] Park BK, Zhang H, et al. NF-kB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF [J]. Nat Med. 2007, 13:62-9.
[4] Huang S, DeGuzman A, et al. Nuclear factor-kB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice [J]. Clin Cancer Res. 2000, 6:2573-81.
[5] Nakshatri H, Bhat-Nakshatri P, et al. Constitutive activation of NF-kappaB during progression of breast cancer to hormone-independent growth [J]. Molecular and cellular biology. 1997, 17:3629-39.
[6] Xiao J,Duan X,et al. The inhibition of metastasis and growth of breast cancer by blocking the NF-κB signaling pathway using bioreducible PEI-based/p65 shRNA complex nanoparticles [J]. Biomaterials. 2013, 34(21):5381-90.
[7] Yanjie Kong, Fubin Li, et al. KHF16 is a Leading Structure from Cimicifuga foetida that Suppresses Breast Cancer Partially by Inhibiting the NF-κB Signaling Pathway [J]. Theranostics. 2016, 6(6):875-86.
[8] Federica Fusella, Laura Seclì, et al. The IKK/NF-κB signaling pathway requires Morgana to drive breast cancer metastasis [J]. Nat Commun. 2017, 8(1):1636.
[9] Zugmaier G, Ennis BW, Deschauer B et al. Transforming growth factors type beta 1 and beta 2 are equipotent growth inhibitors of human breast cancer cell lines [J]. J Cell Physiol. 1989, 141(2): 353–361.
[10] E. M. de Kruijf, T. J. A. Dekker, et al. The prognostic role of TGF-b signaling pathway in breast cancer patients [J]. Ann Oncol. 2013, 24(2):384-90.
[11] King. D, Yeomanson. D, et al. PI3King the Lock: Targeting the PI3K/Akt/mTOR Pathway as a Novel Therapeutic Strategy in Neuroblastoma [J]. Journal of pediatric hematology/oncology. 2015, 37 (4): 245–51.
[12] Atlas N. Comprehensive molecular portraits of human breast tumours[J]. Nature. 2012, 490(7418):61–70.
[13] Raphael, Jacques; Desautels, Danielle. Phosphoinositide 3-kinase inhibitors in advanced breast cancer: A systematic review and meta-analysis [J]. European Journal of Cancer. 2018, 91: 38–46.
[14] Liu T, Yacoub R, et al. Combinatorial efects of lapatinib and rapamycin in triplenegative breast cancer cells [J]. Mol Cancer Ther. 2011, 10(8):1460–1469.
[15] Cossu-Rocca P, Orru S, et al. Analysis of PIK3CA mutations and activation pathways in triple negative breast cancer [J]. PLoS ONE 2015, 10(11):e0141763.
[16] Ooms LM, Binge LC, et al. The inositol polyphosphate 5-phosphatase PIPP regulates AKT1-dependent breast cancer growth and metastasis [J]. Cancer Cell. 2015, 28(2):155–169.
[17] Ricardo L. B. Costa, Hyo Sook Han, et al. Targeting the PI3K/AKT/mTOR pathway in triple‑negative breast cancer: a review [J]. Breast Cancer Res Treat. 2018, 169(3):397-406.
[18] Reedijk M, Odorcic S, et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival [J]. Cancer Res. 2005, 65:8530–7.
[19] Reedijk M, Pinnaduwage D, et al. JAG1 expression is associated with a basal phenotype and recurrence in lymph nodenegative breast cancer [J]. Breast Cancer Res Treat. 2008, 111:439–48.
[20] Hamed Al-Hussaini, Deepa Subramanyam, et al. Notch Signaling Pathway as a Therapeutic Target in Breast Cancer [J]. Mol Cancer Ther. 2011, 10(1):9-15.
[21] Slamon, D.J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene [J]. Science. 1987, 235, 177–182.
[22] Mothaffar F. Rimawi, et al. Targeting HER2 for the Treatment of Breast Cancer [J]. Annu. Rev. Med. 2015, 66:111-28.
[23] L. Gerratanaa, D. Basilea, et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype [J]. Cancer Treat Rev. 2018, 68():102-110.




Comments
Leave a Comment