Calcium-activated potassium channels (KCa) belong to the superfamily of potassium channels. Calcium-activated potassium channels play an important role in regulating vascular membrane potential and tension, as well as regulating smooth muscle cell proliferation. Therefore, it is closely related to many vascular diseases.
1. Classification of Calcium-Activated Potassium Channels
According to the conductance, physiological and pharmacological characteristics of calcium-activated potassium channels, they can be divided into three categories: large conductance calcium-activated potassium channel (BKCa), intermediate conductance calcium-activated potassium channel (IKCa), and small conductance calcium-activated potassium channel (SKCa).
-
Large-Conductance Calcium-Activated K+ Channel: The conductance of BKCa single channel is 100-250 ps. BKCa channels are mainly expressed in smooth muscle cells and neurons, as well as in other cells. The gene was cloned at the slowpork site in Drosophila in 1991. The human gene encoding this channel is located at chromosome 10q22-23. The protein encoded by this gene is a seven-fold transmembrane protein, with the N terminal outside the cell and the C terminal inside the cell. The BKCa channel consists of alpha and beta subunits.
IbTX is the most commonly used BKCa channel blocker. In addition, this channel can also be blocked by charybdotoxin (CTX), tetraethylammonium (TEA), d-tubocurarine and extracellular alkalinization.
Studies have shown that mitoBKCa plays an important role in a variety of physiological and pathological activities such as apoptosis.
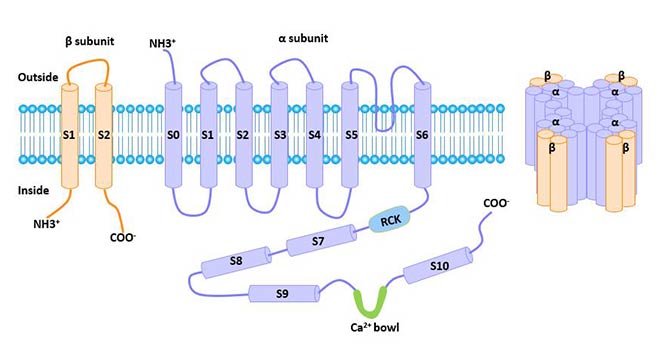
Figure 1 The structure of large conductance calcium-activated potassium channel (BKCa)
-
Small Conductance Calcium-Activated K+ Channel: The unit conductance of SKCa is 4 to 14 ps, which is very sensitive to calcium ions and is non-voltage dependent.
SKCa is mainly expressed in the central system and is also expressed in skeletal muscle cells and human Jurkat-T cells. The gene was isolated from mammalian brain tissue in 1996 and mainly includes SK1, SK2, and SK3.
SKCa can be blocked by apamin or tetrabutylammonium (TBA). Small conductance calcium-activated potassium channels (mitoSKCa) play an extremely important role in cardiovascular diseases [1].
-
Intermediate Conductance Calcium-Activated Potassium Channel: Intermediate conductance Ca2+-activated K+ channel is the KCa3.1 ion channel. The activation of this channel is mainly caused by the increase of intracellular Ca2+ level, which can promote the outflow of K+, thereby regulating the membrane potential and intracellular calcium signal.
Intermediate conductance calcium-activated potassium channels are widely distributed in vascular smooth muscle and are the main outward current potassium channels in vascular smooth muscle.
IKCa can be blocked by CTX, clotrimazole (CLT), TRAM-34, and is not sensitive to apamin.
2. Discovery of Intermediate Conductance Calcium-Activated Potassium Channel
The IKCa gene was cloned from human pancreatic tissue by Ishii and his colleagues in 1997. Its coding gene is KCNN4, located on chromosome 19 (19q13.2). KCNN4, also known as SK4, IK1, KCa4, IKCa1.
3. The Distribution of KCNN4
KCNN4 is expressed in a variety of cells associated with thrombotic diseases, such as smooth muscle cells, T lymphocytes, macrophages, etc. It is also significantly expressed in hematopoietic system cells, salt and fluid transport related organs (including colon, lung, and salivary glands). KCa3.1 channel is known to be expressed in erythrocytes, platelets, lymphocytes, mast cells, monocytes/macrophages, epithelial tissues (gastrointestinal, lung, endocrine glands, exocrine glands), vascular endothelial cells, vascular smooth muscle cells (VSMC), and fibroblasts.
4. The Structure of KCNN
The KCNN gene product is a peptide chain containing 428 amino acids, and the N-terminal and C-terminal of the peptide chain are located in the inner membrane.
The C-terminus is linked to calmodulin, which is a Ca2+ sensor that senses intracellular Ca2+concentration and regulates it [2]. The KCNN gene product contains 6 alpha helical hydrophobic transmembrane S1-S6. There is an inner hydrophobic sequence between S5 and S6 to form the pore region of permeable ions. Pore region is related to the selectivity of K+.
A functional IKCa channel protein is a homotetramer composed of the above peptide chains [3].
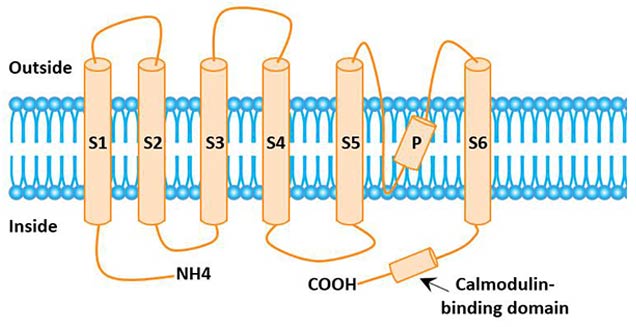
Figure 2 The structural model of IKCa
5. Physiological Effects of Intermediate Conductance Calcium-Activated Potassium Channel
IKCa is a non-voltage-dependent channel and highly sensitive to calcium concentration changes in cytoplasmic, such as growth factor-mediated calcium increase. It is activated by the combination of intracellular Ca2+ and calmodulin [4]. The perception of calcium by IKCa is regulated by calmodulin.
IKCa plays an important role in regulating cell membrane potential and gene expression. The main physiological roles of the KCa3.1 channel are:
-
IKCa channel is involved in the regulation of erythrocyte volume. After the activation of the channel, K+ outflow is accompanied by water loss, making erythrocyte dehydrated and shrivelling [5].
-
IKCa channel can control the Ca2+ inflow and regulate the Ca2+ signal transduction process.
-
IKCa channel is involved in hyperpolarization of endothelial smooth muscle cells, mediating voltage-gated Ca2+ channel closure and causing smooth muscle relaxation [6]. The IKCa channel is also involved in regulating the proliferation of T-lymphocytes and smooth muscle cells. Many vascular diseases, including pulmonary hypertension, systemic hypertension, diabetes and atherosclerosis, are closely related to the IKCa channel.
-
IKCa channel is involved in the secretion process of epithelial cells of digestive tract, lung and secretory gland.
In addition, studies have shown that KCNN4 also plays an important role in cell cycle regulation [7].
6. Regulatory Factors of KCa3.1 Channel
The expression of IKCa is regulated by growth factors such as FGF and transforming growth factor β (TGF-β). For example, in smooth muscle cells, growth factors can regulate calcium signaling and IKCa activity through a protein kinase phosphatase signaling pathway, thereby inducing changes in smooth muscle cell properties [8]. IKCa is also regulated by Ras/MEK/ERK and JAK/STAT signaling pathways.
In addition, an increase in intracellular H+ concentration can reduce the activity of the IKCa channel without affecting its single channel conductance and inward rectification characteristics. The inhibitory effect of H+ did not disappear with the increase of intracellular calcium concentration [9].
At the gene level, the repressor element-1 silencing transcription factor (REST) inhibits the transcriptional level of the KCa3.1 channel [10], while the transcription factor activator protein-1 (AP-1) and Ikaros-2 enhances its transcription level.
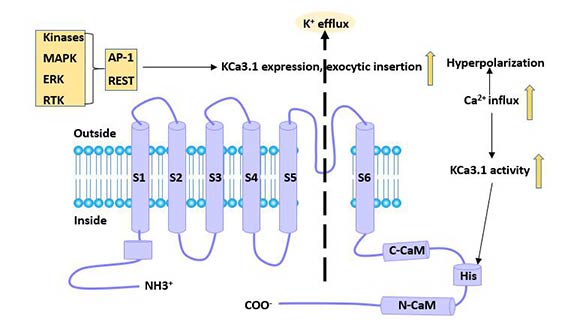
Figure 3 Regulatory factors of KCa3.1 channel
7. KCa3.1 Channels and Thrombotic Disease Research
KCa3.1 is expressed in a variety of cells involved in arteriovenous thrombosis, and regulates cell activation, migration and adhesion by regulating Ca2+ influx and membrane potential levels, thereby participating in the occurrence and development of thrombotic diseases.
The KCa3.1 channel may be involved in thrombotic disease by affecting vascular smooth muscle cells, T lymphocytes, and macrophages. Blocking the KCa3.1 channel can effectively inhibit the progression of arterial plaque and reduce the degree of vascular restenosis, providing a target for the treatment of thrombotic diseases.
7.1 Smooth Muscle Cell KCa3.1 Channel and Thrombotic Disease
Vascular smooth muscle cells (VSMC) have two phenotypes, contractile phenotype and proliferative phenotype. Smooth muscle cells can be plastically transformed between the two phenotypes. Calcium-activated potassium channels are important factors in altering the phenotype of smooth muscle cells. It transforms the vascular smooth muscle from a contractile phenotype to a proliferative phenotype through the coordinated expression of genes.
Under normal conditions, VSMC is contractile phenotype, and the expression of KCa3.1 channel is not detected at this time [11]. When the blood vessel is damaged, the VSMC phenotype is converted and the KCa3.1 channel is expressed.
The mechanism of VSMC proliferation may be: The opening of the KCa3.1 ion channel causes K+ to flow out, causing hyperpolarization of the cell membrane. This leads to an increase in the electrochemical driving force of Ca2+ influx and an increase in intracellular Ca2+ levels, thereby regulating gene expression, protein kinase activation and cell division processes [12] [13]. As an important factor to control vascular smooth muscle hyperplasia, KCa3.1 channel plays a role in the occurrence and development of arterial thrombotic diseases mainly by promoting VSMC proliferation.
7.2 Lymphocyte KCa3.1 Channel and Thrombotic Disease
The voltage-gated potassium channel (Kv channel) and the KCa channel are the two main types of potassium channels present on the lymphocyte membrane. These potassium channels are involved in lymphocyte differentiation, proliferation and activation by modulating intracellular ion concentration and membrane potential.
IKCa is closely related to the activation of T cells. The KCa3.1 channel affects the activation and secretion of T lymphocytes by regulating Ca2+ influx and membrane potential. KCa3.1 channel can participate in the development of arterial thrombosis by regulating T lymphocyte function. Blocking T cell IKCa channels can significantly inhibit T cell proliferation and reduce immune response.
Studies have shown that IKCa can promote the occurrence and progress of ankylosing spondylitis (AS). Inhibition of T cell activation may inhibit the progress of AS. Therefore, IKCa channels can also be used as targets for AS treatment. CLT and TRAM-34 may become anti-AS drugs.
7.3 Macrophage KCa3.1 Channel and Thrombotic Disease
Macrophages are involved in the formation of atherosclerotic plaques. It produces inflammatory factors and oxygen free radicals that promote the progression of plaque [14]. The realization of its function depends mainly on the extent and duration of intracellular Ca2+ increase.
The activation of KCa3.1 channel can promote the Ca2+ influx of macrophages and the hyperpolarization of the cell membrane, thereby regulating the chemotaxis and phagocytosis of macrophages, as well as the production of inflammatory factors and oxygen free radicals. Blocking the KCa3.1 ion channel, the chemotaxis of monocytes/macrophages was significantly reduced.
In addition, cells such as endothelial cells, B lymphocytes, fibroblasts, and platelets are also involved in arteriovenous thrombosis. The activation of KCa3.1 channel is closely related to many functions such as activation and migration of these cells.
8. Calcium-Activated Potassium Channel and Liver Cancer
Liver cancer is a common malignancy of digestive system. It is caused by the unrestricted proliferation and decreased apoptosis of liver cancer cells. Studies have shown that KCNN4 channels regulate cell cycle progression and cell growth of human cancer cells. The proliferation and apoptosis of hepatocellular carcinoma cells are closely related to calcium-activated potassium channels.
Calcium-activated potassium channels are weakly expressed or not expressed in normal cells or tissues, whereas in liver cancer tissues and liver cancer cell lines, their expression levels are abnormally high. This suggests that calcium-activated potassium channels are associated with the development of liver cancer.
Studies have shown that the abnormal expression of calcium-activated potassium channels in hepatocarcinoma may cause tumor cell apoptosis and imbalance of proliferation, formation of tumor blood vessels, cell metastasis and invasion, and have an indirect or direct effect on the progression of liver cancer.
In addition, diseases associated with KCa3.1 ion channels include: sickle cell anemia, inflammation and autoimmune diseases, proliferative diseases, and secretion-related diseases (polycystic kidney).
9. Application of KCa3.1 Channel in Therapeutic Research
KCa3.1 channel is involved in erythrocyte volume regulation, lymphocyte activation, macrophage migration, vascular smooth muscle cell and fibroblast proliferation and other processes through regulating cell membrane potential and calcium signal, and plays a role in the occurrence and development of various diseases.
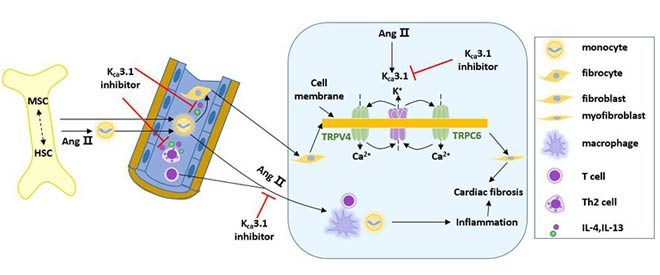
Figure 4 Application of KCa3.1 channel in treatment
Blocking the KCa3.1 channel has a total effect on the treatment of the disease.
-
KCa3.1 channel blockers can block the dehydration of sickle cell, and have now entered the clinical trial phase for the treatment of sickle cell anemia [15].
-
KCa3.1 blocker clotrimazole and its analogs TRAM34 and ICA-17043 inhibit proliferation of cancer cells cultured in vitro [16]. Therefore, KCa3.1 channel blockers are also expected to delay tumor progression [17].
-
Drug blocking or gene knockout of KCa3.1 channel can inhibit fibroblast proliferation and reduce renal fibrosis caused by bilateral ureteral obstruction [18].
-
KCa3.1 channel blockers may be a therapeutic target for chronic renal disease caused by hypertension and diabetes [19].
Wulff et al. [20] developed the KCa3.1 channel blocker TRAM-34 in 2000, which has strong blocking effect and no obvious adverse liver reaction. It is widely used in animal and cell experiments.
The advantage of TRAM-34 is that it is highly selective. For example, the blocking effect on KCa3.1 channels is more than 1000 times that of other potassium channels, sodium channels and calcium channels; TRAM-34 does not reduce the cell activity of human T lymphocytes, VSMC, macrophages and endothelial cells [21].
References
[1] Xu W. Cytoprotective Role of Ca2+ - Activated K+ Channels in the Cardiac Inner Mitochondrial Membrane [J]. Science (Washington D C), 2002, 298(5595): 1029-1033.
[2] Fanger C M, Ghanshani S, Logsdon N J, et al. Calmodulin Mediates Calcium-dependent Activation of the Intermediate Conductance KCa Channel, IKCa1 [J]. Journal of Biological Chemistry, 1999, 274(9): 5746-5754.
[3] Maher A D, Kuchel P W. The Gardos channel: a review of the Ca2+-activated K+ channel in human erythrocytes [J]. The International Journal of Biochemistry & Cell Biology, 2003, 35(8).
[4] Ishii T M, Silvia C, Hirschberg B, et al. A Human Intermediate Conductance Calcium-Activated Potassium Channel [J]. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(21): 11651-11656.
[5] Ataga K I. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia [J]. Blood, 2008, 111(8): 3991.
[6] Sebastian Br?hler, Kaistha A, Schmidt V J, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension [J]. Circulation, 2009, 119(17): 2323-2332.
[7] Lallet-Daher H, Roudbaraki M, Bavencoffe A, et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry [J]. Oncogene, 2009, 28(15): 1792-1806.
[8] Pena T L. Ras/MEK/ERK Up-regulation of the Fibroblast KCa Channel FIK Is a Common Mechanism for Basic Fibroblast Growth Factor and Transforming Growth Factor-beta Suppression of Myogenesis [J]. Journal of Biological Chemistry, 2000, 275(18): 13677-13682.
[9] Gerlach A C. ATP-dependent Activation of the Intermediate Conductance, Ca2+-activated K+ Channel, hIK1, Is Conferred by a C-terminal Domain [J]. Journal of Biological Chemistry, 2001, 276(14): 10963-10970.
[10] Cheong A, Bingham A J, Li J, et al. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation [J]. Molecular Cell, 2005, 20(1): 45-52.
[11] Tharp D, Bowles D. The Intermediate-Conductance Ca2+-Activated K+ Channel (KCa3.1) in Vascular Disease [J]. Cardiovascular & Hematological Agents in Medicinal Chemistry, 2009, 7(1): 1-11.
[12] Ghanshani S, Wulff H, Miller M J, et al. Up-regulation of the IKCa1 Potassium Channel during T-cell Activation: MOLECULAR MECHANISM AND FUNCTIONAL CONSEQUENCES [J]. Journal of Biological Chemistry, 2000, 275(47): 37137-37149.
[13] Stevenson A S, Gomez M F, Hill-Eubanks D C, et al. NFAT4 Movement in Native Smooth Muscle: A ROLE FOR DIFFERENTIAL Ca2+ SIGNALING [J]. Journal of Biological Chemistry, 2001, 276(18): 15018-15024.
[14] Lucas A R, Korol R, Pepine C J. Inflammation in Atherosclerosis [J]. J Assoc Physicians India, 2006, 48(2): 265-266.
[15] Brugnara C, Franceschi L D. Clinical trials of new therapeutic pharmacology for sickle cell disease [J]. Sante, 2006, 16(4): 263-268.
[16] Chou C C, Lunn C A, Murgolo N J. KCa3.1: target and marker for cancer, autoimmune disorder and vascular inflammation? [J]. Expert Review of Molecular Diagnostics, 2008, 8(2): 179-187.
[17] Jager H. Blockage of Intermediate-Conductance Ca2+-Activated K+ Channels Inhibit Human Pancreatic Cancer Cell Growth in Vitro [J]. Molecular Pharmacology, 2004, 65(3): 630-638.
[18] Grgic I, Kiss E, Kaistha B P, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels [J]. Proceedings of the National Academy of Sciences, 2009, 106(34): 14518-14523.
[19] Huang C, Pollock C A, Chen X M. KCa3.1: A new player in progressive kidney disease [J]. Current Opinion in Nephrology and Hypertension, 2014, 24(1).
[20]Wulff H, Castle N A. Therapeutic potential of KCa3.1 blockers: recent advances and promising trends [J]. Expert Review of Clinical Pharmacology, 2010, 3(3): 385.
[21]Toyama K, Wulff H, Chandy K G, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans [J]. Journal of Clinical Investigation, 2008, 118(9): 3025-3037.
CUSABIO team. Information You Must Know about KCNN4. https://www.cusabio.com/c-20939.html








Comments
Leave a Comment