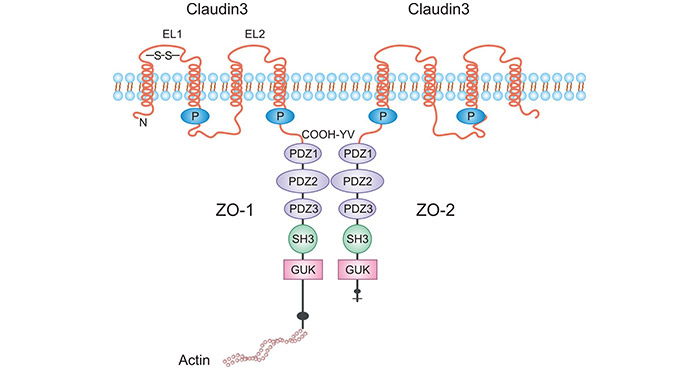
[1] Fujiwara, Sachiko, et al. "Tight junction formation by a claudin mutant lacking the COOH-terminal PDZ domain-binding motif ." Annals of the New York Academy of Sciences 1516.1 (2022): 85-94.
[2] Castro Dias, Mariana, et al. "Claudin-3-deficient C57BL/6J mice display intact brain barriers." scientific reports 9.1 (2019): 203.
[3] Che, J., Yue, D., et al. "Claudin-3 Inhibits Lung Squamous Cell Carcinoma Cell Epithelial-mesenchymal Transition and Invasion via Suppression of the Wnt/β-Catenin Signaling Pathway." International Journal of Medical Sciences, 15(4) (2018): 339-351.
[4] Lei, Ningjing, et al. "Claudin-3 inhibits tumor-induced lymphangiogenesis via regulating the PI3K signaling pathway in lymphatic endothelial cells. " Scientific Reports 12.1 (2022): 17440.
[5] Wang, ZhaoHan, et al. "Plasma claudin-3 is associated with tumor necrosis factor-alpha-induced intestinal endotoxemia in liver disease." Clinics and Research in Hepatology and Gastroenterology 43.4 (2019): 410-416.
[6] Yang, Y., et al. "Mo1775 Epigenetic Inactivation of Claudin 3 in Hepatocellular Carcinoma and Its Functional Consequences." Gastroenterology 5.144 ( 2013): S-1023.
[7] Torres, Julia Baguña, et al. "Radiolabeled cCPE peptides for SPECT imaging of claudin-4 overexpression in pancreatic cancer." journal of Nuclear Medicine 61.12 (2020): 1756-1763.
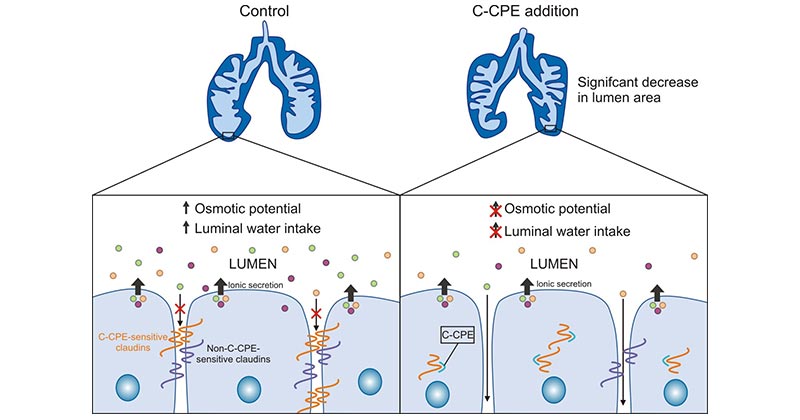
[8] La Charité-Harbec, Simon, et al. "Claudin-3 regulates luminal fluid accumulation in the developing chick lung." Differentiation 124 (2022): 52-59.
[9] Li, Xiangru, et al. "Development of an Anti-Claudin-3 and -4 Bispecific Monoclonal Antibody for Cancer Diagnosis and Therapy." journal of Pharmacology and Experimental Therapeutics 351.1 (2014): 206-213.
[10] Pahle, Jessica, et al. "Effective oncoleaking treatment of pancreatic cancer by claudin-targeted suicide gene therapy with Clostridium perfringens enterotoxin (CPE)." Cancers 13.17 (2021): 4393.
[11] Gasperoni, Jemma G., et al. "Grainyhead-like (Grhl) target genes in development and cancer." International Journal of Molecular Sciences 23.5 (2022): 2735.
[12] Ahmad, Rizwan, et al. "Loss of claudin-3 expression induces IL6/gp130/Stat3 signaling to promote colon cancer malignancy by hyperactivating Wnt/β- catenin signaling." Oncogene 36.47 (2017): 6592-6604.
[13] Reiling, Janske, et al. "Low-Dose Lipopolysaccharide Causes Biliary Injury by Blood Biliary Barrier Impairment in a Rat Hepatic Ischemia /Reperfusion Model." Liver Transplantation 23.2 (2017): 194-206.
[14] de Souza, Waldemir F., et al. "Claudin-3 overexpression increases the malignant potential of colorectal cancer cells: roles of ERK1/2 and PI3K-Akt as modulators of EGFR signaling." ploS one 8.9 (2013): e74994.
[15] Wang, Yaxi, et al. "SCF/C-Kit/JNK/AP-1 signaling pathway promotes claudin-3 expression in colonic epithelium and colorectal carcinoma." International journal of molecular sciences 18.4 (2017): 765.
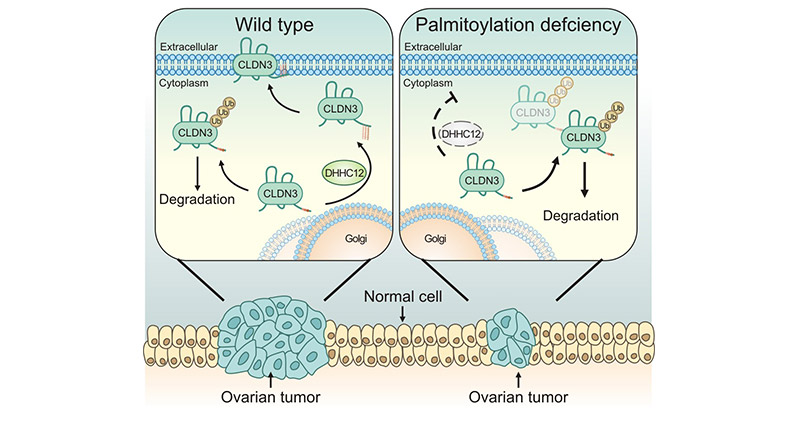
[16] Yuan, Meng, et al. "ZDHHC12-mediated claudin-3 S-palmitoylation determines ovarian cancer progression." Acta Pharmaceutica Sinica B 10.8 (2020): 1426-1439.
[17] Che, Juanjuan, et al. "Decreased expression of claudin-3 is associated with a poor prognosis and EMT in completely resected squamous cell lung carcinoma ." Tumor Biology 36 (2015): 6559-6568.
[18] Orea, María J., et al. "Claudin-3 Loss of Expression Is a Prognostic Marker in Castration-Resistant Prostate Cancer." International Journal of Molecular Sciences 24.1 (2023): 803.
[19] Tessier-Cloutier, Basile, et al. "Proteomic analysis of transitional cell carcinoma-like variant of tubo-ovarian high-grade serous carcinoma." Human pathology 101 (2020): 40-52.
[20] Chen, Peng, et al. "Structural basis for CSPG4 as a receptor for TcdB and a therapeutic target in Clostridioides difficile infection." Nature communications 12.1 (2021): 3748.
[21] Xie, Xiaoxi, et al. "Roles of gastrointestinal polypeptides in intestinal barrier regulation." Peptides (2022): 170753.
[22] Liu, Jinhui, et al. "Development of an immune gene prognostic classifier for survival prediction and response to immunocheckpoint inhibitor therapy/ chemotherapy in endometrial cancer." International immunopharmacology 86 (2020): 106735.
[23] Szade, Jolanta, et al. "Comparison of claudin-3 and claudin-4 expression in bilateral and unilateral breast cancer." Neoplasma 68.2 (2020): 283-289.
[24] Feng, Ji, et al. "Capsaicin inhibits migration and invasion via inhibiting epithelial-mesenchymal transition in esophageal squamous cell carcinoma by up-regulation of claudin-3 expression." Journal of Functional Foods 89 (2022): 104934.
[25] Che, Juanjuan, et al. "Claudin-3 inhibits lung squamous cell carcinoma cell epithelial-mesenchymal transition and invasion via suppression of the Wnt /β-catenin signaling pathway." international journal of medical sciences 15.4 (2018): 339.
[26] Haseloff, Reiner F., et al. "Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects ." Seminars in cell & developmental biology. vol. 38. Academic Press, 2015.
[27] Tanaka, Hiroo, et al. "Claudin-3 regulates bile canalicular paracellular barrier and cholesterol gallstone core formation in mice." journal of hepatology 69.6 (2018): 1308-1316.
[28] Baier, Felix Alexander, et al. "Loss of Claudin-3 Impairs Hepatic Metabolism, Biliary Barrier Function, and Cell Proliferation in the Murine Liver." Cellular and molecular gastroenterology and hepatology 12.2 (2021): 745-767.




Comments
Leave a Comment