Antibodies are indispensable tools in biological research, medicine, and various industrial applications. Highly pure antibodies give rise to reliable experimental outcomes and rigorous clinical data, ensuring safety and quality in various applications. High-purity antibodies are thus pursued and longed for by researchers and medical doctors. Therefore, the significance of antibody purification cannot be overstated.
This article explores the diverse array of antibody purification methods available to researchers, focusing on their advantages, limitations, and suitability for different research needs. By understanding the nuances of each purification method, researchers can make informed decisions to optimize their antibody purification protocols according to the specific requirements of their projects.
Table of Contents
1. What Is Antibody Purification?
Antibody purification is the process of extracting and enriching antibodies from a complex mixture, such as antiserum, ascites fluid, or hybridoma cell culture supernatant, which is vital in ensuring the reliability and effectiveness of downstream experiments.
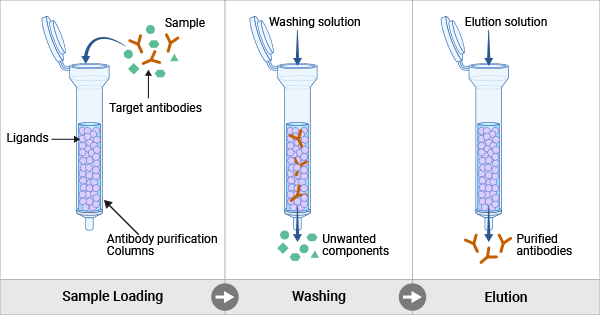
Figure 1. Antibody purification through affinity chromatography
2. Why Is Antibody Purification Important?
Natural antibodies are usually isolated from antiserum or cell culture supernatant, which may contain various contaminants, such as host cell proteins, DNA, polysaccharides, lipids, etc. These impurities may affect the purity, activity, and specificity of the antibody. The significance of antibody isolation and purification is mainly reflected in the following aspects:
2.1 Improve antibody purity
Purification of the antibody-containing solution can remove the majority of impurities, thus improving the purity of the antibody. High-purity antibodies can improve their specificity and activity and reduce non-specific binding, cross-reactivity, and background interference, which improves the accuracy and reliability of experimental results.
2.2 Guarantee antibody quality
The antibody purification process can also remove harmful substances, such as protease, endotoxin, etc., guaranteeing the quality and safety of the antibody.
In therapeutic applications, impurities can cause unwanted immune reactions. Highly pure antibodies minimize the risk of immunogenic responses in patients. High purity ensures that the administered dose is predominantly active antibody, enhancing therapeutic efficacy. The therapeutic effectiveness of antibodies can be compromised by impurities. High purity ensures that the administered dose is predominantly active antibody, enhancing therapeutic efficacy.
2.3 Ensure antibody consistency
Purification protocols allow for the extraction of homogeneous antibody populations, ensuring consistency between different batches or experiments. Consistent antibody performance is essential for standardizing experimental procedures and achieving reproducible results across multiple studies or assays.
2.4 Enhance antibody stability
The purified antibody is not susceptible to external factors, such as temperature, pH, etc., retaining stabler in the storage and use process. This helps to extend the shelf life and service life of the antibody.
In summary, antibody purification is important for obtaining highly pure, specific, and active antibodies, which is of great significance for biomedical research, diagnosis, and treatment.
3. How to Purify Antibodies
Antibody purification slightly varies from different antibody types. The general antibody purification is a multi-step process, involving sample preparation, clarification, moderate purification, and fine purification.
3.1 Sample Preparation
Antibodies are generated from native or recombinant sources. Sample preparation is the initial step and involves collecting the source material containing the antibodies, such as serum, cell culture supernatant, or ascites fluid.
3.2 Clarification
Clarification is a preliminary extraction process that removes lipids and particle matter such as cellular debris to separate the antibody-containing supernatant from the collected sample. Centrifugation is often used to clarify the sample, thus obtaining a crude extract enriched with antibodies. Additional filtration steps can further clarify the sample.
3.3 Capture
After clarification, the obtained crude extraction is subjected to the capture step for further decontamination. The capture step is the first purification. The antibody-containing sample is usually loaded onto the purification column, where the target antibodies are selectively captured by the matrix’s ligands or charged groups bound on the column while unwanted components pass through as flow-through.
During the capture step, antigen-specific capture methods or Protein A/G affinity chromatography are selected for monoclonal antibodies. Protein A/G or Protein L affinity chromatography is usually used to capture polyclonal antibodies or recombinant antibodies. Capture for tagged recombinant antibodies is also captured by ligands specific for their co-expressed affinity tags. Immobilized metal affinity chromatography (IMAC) is utilized for purifying recombinant antibodies that are expressed with polyhistidine tags.
After capture, elution of the bound antibodies from the column using a low pH buffer or a buffer containing high concentrations of salt or chaotropic agents. Collect the eluate containing the purified antibodies.
There are additional purification methods, which will be described in detail in point 4 of the text.
3.4 Polishing
Polishing, also called fine purification, is not a mandatory step, where the isolated antibodies undergo rigorous purification to achieve higher purity. Several techniques such as ion exchange chromatography, hydrophobic interaction chromatography, size exclusion chromatography, high-performance liquid chromatography (HPLC), and fast protein liquid chromatography (FPLC) are used for polishing antibodies.
3.5 Analysis and Storage
Throughout the purification process, various analytical techniques such as SDS-PAGE, ELISA, mass spectrometry, or UV spectroscopy are employed to assess the purity, concentration, integrity, and functionality of the isolated antibodies. Aliquot the purified antibodies into smaller volumes. Store the purified antibodies in appropriate buffers and conditions to maintain their stability and activity.
4. Methods for Antibody Purification
There are several methods for purifying antibodies, each with its advantages and suitability depending on the type of antibody and the intended downstream applications.
Table 1. Commonly used antibody purification techniques
| Methods |
Mechanism |
Advantages |
Disadvantages |
| Affinity Chromatography |
Protein A, protein G, protein L, and protein A/G affinity chromatography |
Protein A, protein G, protein L, and protein A/G selectively bind to antibodies [1,2] |
High affinity for immunoglobulins;
Scalable for large-scale purification;
High resolution;
High speed;
Suitable for monoclonal and polyclonal antibody purification |
High cost od protein A/G/L resins;
Low pH elution (protein A) [3-5];
Protein A leaching and toxicity [5] |
| Antigen Affinity Purification |
Antigen-antibody specific interaction [1] |
High specificity;
Scalable for large-scale purification;
High resolution;
High speed;
Suitable for polyclonal antibody purification |
Needs a large amount of specific antigens, increasing coast |
| Size Exclusion Chromatography (SEC)
| Purifies antibodies based on their molecular size [6] |
Gentle purification; preserves antibody activity;
Suitable for the purification of monoclonal antibodies and antibody drg conjugates (ADCs) |
Lower resolution compared to affinity chromatograph;
Limited binding capacity;
Low efficiency |
| Ion Exchange Chromatography (IEC)
| Separation based on the net surface charge of antibodies, which are captured by the oppositely charged groups on the solid matrix [7] |
Good reproducibility, simple and easy to operate;
High resolution and high recovery rate;
High throughput purification;
Conditions are mild, which will not cause antibodys denaturation or inactivation; Widely used for the purification of monoclonal antibodies |
Requires optimization for pH and ionic strength;
May cause antibody aggregation |
| Hydrophobic Interaction Chromatography (HIC)
| Antibodies selectively interacts with the hydrophoic ligands on the high-salt buffer-equilibrated HIC column based on their hydrophobicity [8] |
Good resolution;
High volume purification;
High speed;
Scalability;
Mild conditions preserve the native structure and biological activity of antibodies;
Suitanle for polyclonal and recombinant antibody purification |
May exhibit non-specific binding with certain proteins, causing contamination;
Later optimization is time-consuming and require extensive experimentation |
| Precipitation
| Antibody solubility in specific conditions [9] |
Simple and cost-effective method;
High yield;
Suitable for large-scale purification |
Risk of antibody denaturation and aggregation |
These methods offer various advantages and disadvantages, as well as suitability for the purification of different types of antibody.
5. How to Select the Appropriate Antibody Purification Method
Whichever the type of antibodies, selecting an appropriate purification strategy will allow isolating target antibodies with high purity following production successfully. Here are several key factors to ensure successful purification:
5.1 Type of Antibodies
Consider the antibody type (monoclonal, polyclonal, or recombinant), tagged or not, antibody isotype, and the species of origin. Different purification methods may be more effective for certain types of antibodies.
5.2 Compatibility
Ensure the chosen purification method is compatible with the sample matrix and any additives or buffers used in sample preparation.
5.3 Purity Requirements
Determine the desired purity level for the purified antibodies based on the intended downstream applications. Antibodies with higher purity are required for therapeutic applications compared to research purposes. Diagnostic assays may require higher purity compared to preliminary research experiments.
5.4 Intrinsic Physical and Chemical Properties of Antibodies
Consider the antibodies’ specificity and affinity for antigen and protein A/G/L, charge properties, stability, activity, etc. Evaluate the specificity of the antibodies and whether purification based on antigen-antibody interactions or other specific binding properties is necessary. Choose the method that maintains the antibody stability and does not impair antibody functionality.
5.5 Downstream Applications
Consider the intended use of the purified antibodies, such as ELISA, WB, IHC, therapeutic applications, or structural studies. Some purification methods may be better suited for specific applications.
5.6 Cost and Resources
Assess the cost-effectiveness of different purification methods, including the availability of equipment, consumables, and expertise required for each method. Consider cost-effective methods that fit within the budget.
5.7 Processing Time
Consider the time constraints for purification, including the duration of the purification process and the time required for method optimization and validation.
By carefully considering these factors, researchers can select the appropriate antibody purification method to meet their specific research needs and obtain purified antibodies of the desired quality and quantity. Additionally, conducting pilot experiments or feasibility studies may help compare and optimize different purification methods before proceeding with large-scale purification.
Conclusion
Antibody purification is important in antibody and biotechnology research and is widely used in medicine, drug development, and life sciences. Antibody purification is a multi-step process involving antibody production, sample pretreatment, and the application of various purification techniques for decontamination. We can obtain antibodies with high purity and strong activity through reasonable selection and combination of these purification methods, which provide reliable experimental materials for subsequent experiments.
References
[1] Roque AC, Silva CS, Taipa MA. Affinity-based methodologies and ligands for antibody purification: advances and perspectives [J]. J Chromatogr A. 2007 Aug 10;1160(1-2):44-55.
[2] Huse K, Bohme HJ, Scholz GH. Purification of antibodies by affinity chromatography [J]. J Biochem Biophys Methods . 2002;51(3):217–31.
[3] Arakawa, T.; Philo, J.S.; et al. Elution of antibodies from a protein-A column by aqueous arginine solutions [J]. Protein Expr. Purif. 2004, 36, 244–248.
[4] Carter-Franklin, J.N.; Victa, C.; et al. Fragments of protein A eluted during affinity chromatography [J]. J. Chormatogra. A 2007, 1163, 105–111.
[5] Arakawa, T.; Tomioka, Y.; et al. Non-Affinity Purification of Antibodies [J]. Antibodies 2023, 12, 15.
[6] Ackers GK. Molecular exclusion and restricted diffusion processes in molecular-sieve chromatography [J]. Biochemistry. 1964;3(5):723-730.
[7] Liu HF, Ma J, et al. Recovery and purification process development for monoclonal antibody production [J]. MAbs. 2010 Sep-Oct;2(5):480-99.
[8] Pereira Bresolin IRA, Lingg N, et al. Hydrophobic interaction chromatography as polishing step enables obtaining ultra-pure recombinant antibodies [J]. J Biotechnol. 2020;324S:100020.
[9] Z. Li, Q. Gu, J.L. Coffman, et al. Continuous precipitation for monoclonal antibody capture using countercurrent washing by microfiltration [J]. Biotechnol. Prog., 35 (2019), p. e2886.
CUSABIO team. What Are the Best Antibody Purification Methods for Your Research Needs?. https://www.cusabio.com/c-21176.html



Comments
Leave a Comment