Are you struggling to decide which type of monoclonal antibody to choose?
Then you need to understand the source of monoclonal antibody - monoclonal antibody technology.
This guide explains what monoclonal antibody technology is, why it is important, and how to choose the most appropriate monoclonal antibody technology for your monoclonal antibodydevelopment.
Table of Contents
1. What Is Monoclonal Antibody Technology?
Monoclonal antibody technology refers to methods that produce monoclonal antibodies (mAbs). Monoclonal antibodies are produced by identical B cells and characterized by superior specificity and high consistency. They are invaluable for a wide range of applications, from research reagents and disease diagnostics to targeted cancer therapies.
Monoclonal antibody technology differs significantly from polyclonal antibody production, where antibodies are derived from different B cells and target multiple epitopes on an antigen.
2. Why Is Monoclonal Antibody Technology Important in Science Research and Medicine?
Monoclonal technology is critically important in scientific research and medicine because its end-products monoclonal antibodies possess unique properties that allow for high specificity, precision targeting, and versatility.
Monoclonal antibodies are vital tools in research for studying protein functions, signaling pathways, and cellular processes. They help to elucidate the role of different molecules in health and disease by blocking or activating specific targets. Also, monoclonal antibodies are often used to screen and identify potential drug candidates.
Monoclonal antibodies are also essential in diagnostics and therapeutics. They can be used in immunoassays such as ELISA and WB to detect and identify specific proteins, hormones, and pathogens, enabling early and accurate disease detection, which is critical for effective treatment. In therapeutics, monoclonal antibodies have revolutionized the treatment of various diseases, particularly in oncology. They are also made as antibody-drug conjugates (ADCs) that can deliver these drugs specifically to cancer cells while minimizing damage to normal cells.
3. Types of Monoclonal Antibody Technology
Hybridoma technology is the earliest pioneered monoclonal antibody technology. With the increasing demand for therapeutic antibodies, phage display, single B cell, transgenic animal models, and other monoclonal antibody technologies have been derived successively.
3.1 Hybridoma Technology
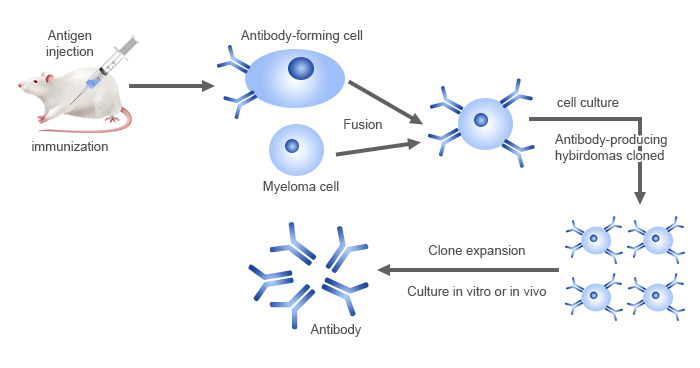
Hybridoma technology is the classical and most widely used method for producing monoclonal antibodies. Pioneered in 1975 by Georges Köhler and César Milstein [1], hybridoma technology involves fusing an antigen-specific B-cell with a myeloma cell to create a hybridoma that can be cultured indefinitely while producing a single type of antibody.
Figure 1. Monoclonal antibody production workflow by hybridoma technology
Hybridoma technology is mainly used to produce murine monoclonal antibodies, which commonly serve as scientific research reagents. Murine mAbs can be further chimeric or humanized for therapeutic purposes by combining their variable regions or complementary determining regions (CDRs) with human constant regions, respectively, through antibody engineering techniques [5,6]. Hybridoma technology can also be used to generate fully human antibodies in transgenic mice models [7].
Pros:
- High specificity and purity
- Long-term production capability
- Well-established and widely used
Cons:
- Time-consuming and labor-intensive
- Generation of human anti-mouse antibody response (HAMA) due to high immunogenicity of the murine-derived mAbs [2]
- Limited antibody diversity
Further reading: Monoclonal Antibody Production through Hybridoma Technology
3.2 Phage Display Technology
Phage display technology was first described in 1985 by George P. Smith for the display of peptides [3] and later, first used to display antibody fragments on phages in 1990 by McCafferty and Winter [4]. This method can construct phage display libraries using bacteriophages to display different antibody formats, from which specific antibodies were identified by a specific antigen.
Figure 2. Monoclonal antibody production through phage display technology
Phage display technology is often used for the discovery of mAbs for research, diagnostic, and therapeutic applications, including single-chain variable fragments (scFvs), antigen-binding fragments (Fabs), and fully human monoclonal antibodies. This technique also allows for the engineering of antibody fragments with optimized properties such as enhanced affinity or specificity, making them suitable for use in antibody-drug conjugates (ADCs) and bispecific antibodies.
Pros:
- Ability to screen large library
- Flexibility in selecting different antibody formats
Cons:
- Requires specialized knowledge and equipment
- Potential for lower yield compared to hybridoma technology
Further reading: Monoclonal Antibody Production through Phage Display Technology
3.3 Single B Cell Technology
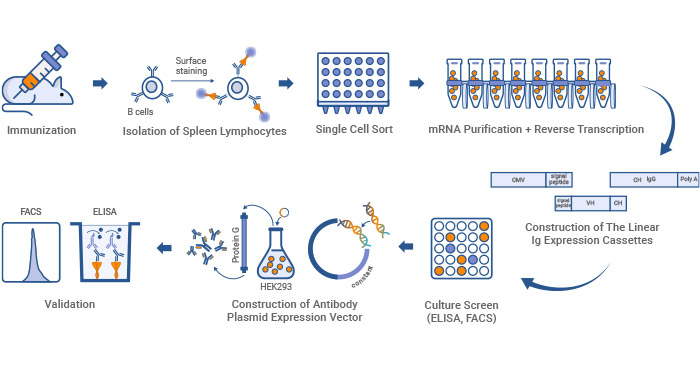
Single B cell technology is a cutting-edge method that isolates and characterizes monoclonal antibodies directly from individual B-cells. It mainly involves the amplification of the genes encoding the variable heavy (VH) and variable light (VL) region of immunoglobulin (Ig) derived from single B cells.
Figure 3. Monoclonal antibody production workflow by single B cell technology
Single B cell technology is primarily used to produce fully human mAbs, which are particularly valuable in therapeutic applications.
Pros:
- Preservation of the natural VH and VL pairing
- Conservation of natural diversity of the antibody repertoire
- Rapid production of fully human mAbs
Cons:
- Technically complex and requires advanced equipment
- The early stages are time-consuming
Further reading: Monoclonal Antibody Production through Single B Cell Technology
3.4 Transgenic Animal Models
Transgenic animal platforms use the natural recombination and affinity machinery to produce human mAbs of wide diversity and high affinity. This technology was introduced by the publication of two transgenic mouse lines, the HuMabMouse [8] and the XenoMouse [9].
In the transgenic approach, the human Ig genes are inserted into the animal’s genome to replace the endogenous Ig genes, rendering the animal capable of generating fully human mAbwsw upon immunization [10]. The process of producing monoclonal antibodies using transgenic mice is similar to the mouse hybridoma technique. The primary application of fully human mAbs from transgenic animals is in therapeutics.
Pros:
- Production of fully human antibodies
- No need for humanization or deimmunization required
- More diversity
- In vivo affinity maturation and clonal selection for antibody optimization
Cons:
- The large size of the human Ig loci
- Ethical considerations
- Access to transgenic technology is difficult and expensive
4. How to Choose Monoclonal Antibody Technology?
Selecting the appropriate monoclonal antibody technology depends on several factors, including your specific application, required antibody characteristics, production scalability, humanization and immunogenicity, and budget. Here are some steps to guide your decision:
4.1 Define Your Application Purpose
The intended use of the monoclonal antibody significantly influences the choice of production technology. Are you developing a therapeutic antibody, a diagnostic tool, or conducting basic research? The antibody produced by hybridoma technology is often for a scientific experiment. For therapeutic applications, technologies like phage display or transgenic animal models that can produce fully human antibodies are preferable to minimize immunogenicity.
4.2 Consider Specificity and Affinity Requirements
Different applications require different levels of antibody specificity and affinity. For therapeutic applications, high specificity is critical to avoid off-target effects. In contrast, diagnostic applications may prioritize affinity to ensure sensitive detection of low-abundance antigens. Phage display or single B cell technologies are more suitable for these types of applications. Phage display is often used to develop monoclonal antibodies for cancer therapy, which is crucial to ensure antibodies to precisely target cancer cells minimizing affecting normal tissues.
4.3 Evaluate Production Scalability
Consider the scalability of the technology. Hybridoma technology is well-suited for large-scale production and is ideal for generating diagnostic monoclonal antibodies used in commercial ELISA kits.
4.4 Humanization and Immunogenicity
Human therapeutic monoclonal antibodies are commonly fully human or humanized antibodies, which is critical to reducing the risk of immune rejection. Transgenic animal models are often used when fully human antibodies are required.
4.5 Budget and Time Constraints
Finally, consider your budget and timeline. The time required for development and the associated costs vary greatly between technologies. Single B cell technology offers repaid development of fully human antibodies, making it ideal for urgent therapeutic projects, though it may be expensive initially compared to hybridoma technology.
Conclusion
Choosing the right monoclonal antibody technology is critical for the success of your project. By understanding the various technologies available and aligning them with your specific needs, you can make an informed decision that enhances the precision, effectiveness, and scalability of your work. Whether you’re focused on therapeutic development, diagnostic innovation, or research, the right choice of monoclonal antibody technology will pave the way for groundbreaking discoveries and applications.
References
[1] Köhler, G., and Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity [J]. Nature 256, 495–497.
[2] Hwang, W. Y. K., and Foote, J. (2005). Immunogenicity of engineered antibodies [J]. Methods 36, 3–10.
[3] Smith, G. P. (1985). Filamentous fusion phage: novel expression vectors that display cloned antigens on the Virion surface [J]. Science 228, 1315–1317.
[4] McCafferty, J., Griffiths, et al. (1990). Phage antibodies: filamentous phage displaying antibody variable domains [J]. Nature 348, 552–554.
[5] Studnicka GM, Soares S, et al. Human-engineered monoclonal antibodies retain full specific binding activity by preserving non-CDR complementarity-modulating residues [J]. Protein Eng. (1994) 7:805–14.
[6] Jones PT, Dear PH, et al. Replacing the complementarity-determining regions in a human antibody with those from a mouse [J]. Nature. (1986) 321:522–5.
[7] Lonberg N. Human antibodies from transgenic animals [J]. Nat Biotechnol. (2005) 23:1117–25.
[8] Lonberg N, Taylor LD, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications [J]. Nature. 1994;368:856–9.
[9] Mendez MJ, Green LL, et al. Functional transplant of megabase human immunoglobulin loci recapitulates human antibody response in mice [J]. Nat Genet. 1997;15:146–56.
[10] Green LL, Hardy MC, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs [J]. Nat Genet. 1994;7:13–21.
CUSABIO team. How to Choose Monoclonal Antibody Technology? A Definitive Guide. https://www.cusabio.com/c-21189.html





Comments
Leave a Comment