Selecting the appropriate isotype control antibodies is a critical decision in experimental design, yet it often gets considerably less focus than other research components. For many researchers, isotype control is merely an afterthought, added to an experiment with insufficient understanding of its crucial role in data interpretation. Using an incorrect isotype control or omitting one entirely can result in misleading findings and lead to significant errors in conclusions.
In this article, we will dive into the importance of isotype control antibodies, explore their mechanisms of action and advantages, and guide you in choosing the most appropriate isotype control for your experiment.
Table of Contents
1. What Is An Isotype Control?
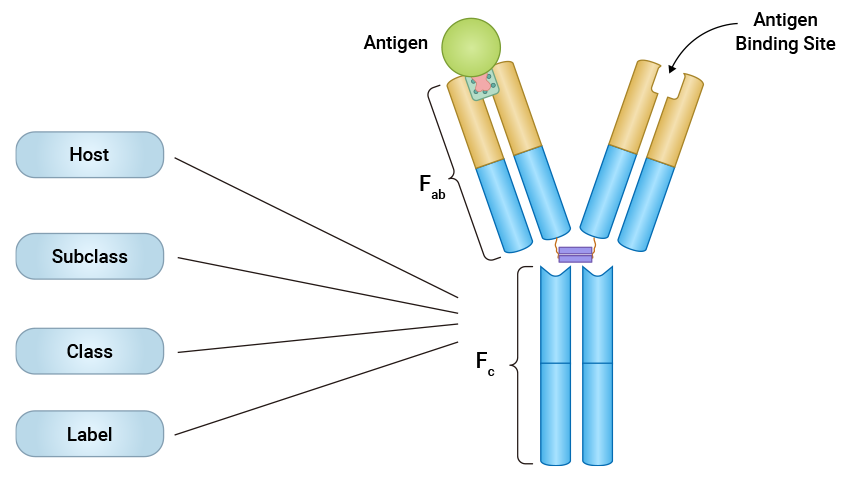
An isotype control is an antibody used as a negative control to differentiate the specific-antibody staining and non-specific background staining in immunological experiments. Isotype control antibodies should match the host species, isotype, and subclass of the test/primary antibody but not specific to the target antigen. Isotype control antibodies are usually developed against antigens not present in the cell type or sample being analyzed, so they can not react with the target antigens.
Figure 1. Isotype control criteria
2. Why Is It Important to Use An Isotype Control?
Generally, all antibodies exhibit a high similarity in structure. An antibody molecule is typically composed of the "constant region" where the amino acid sequence of antibodies of the same isotype is identical and the variable region that determines specific binding to antigens. The constant region is important for mediating the antibody's mechanism of action (MOA) by binding to Fc receptors present on effector cells.
When conducting antibody-based assays, it is essential to control for any variables that may affect the interpretation of the research results. The non-antigen-specific Fc receptor engagement can produce detectable biological effects or phenotypes not associated with antigen-specific binding [1]. A proper isotype control antibody will also interact with Fc receptors, enabling the researcher to account for these additional variables and facilitating more precise and reliable data analysis.
Without an isotype control, the experimental results may be confounded by several factors that can cause misinterpretations of data resulting in inaccurate conclusions of the antibody research.
3. Advantages of Using An Isotype Control
Researchers can evaluate the level of non-specific background by conducting a control experiment alongside the main experiment, replacing the primary antibody with isotype control, while keeping all other conditions, such as concentration, buffers, and temperature, identical. Here are several advantages of incorporating an isotype control.
3.1 Identify Non-Specific Binding
The isotype control mainly functions to distinguish between specific and non-specific binding. All antibodies have some degree of non-specific binding because of interactions with Fc receptors on cells or non-specific charge interactions with components in the sample. An isotype control helps quantify this "background" signal, ensuring that the observed binding is a result of the primary antibody rather than these non-specific interactions.
3.2 Improve Data Interpretation
In techniques like flow cytometry, background staining can hinder data interpretation. Failing to use an isotype control makes it challenging to differentiate between the actual signal and unwanted background noise. By comparing the signal obtained from the experimental antibody to that of the isotype control, researchers can discern which portions of the signal are specific and which are background noise.
3.3 Validate Experimental Conditions
Isotype controls also serve as a tool to validate that the experimental conditions are appropriate. If the isotype control shows a significant signal, it indicates potential problems with the experimental setup, like too much antibody concentration or poor blocking procedures. The isotype control can act as a safeguard to verify the reliability of your results and the proper functioning of the assay.
3.4 Standardization and Reproducibility
Isotype controls ensure that results are both accurate and consistent across experiments. By employing an isotype control, researchers can standardize their procedures, making it simpler to replicate experiments and share data with the scientific community.
An untreated group or PBS-treated group can not be used in place of an isotype control-treated group.
4. How Do Isotype Controls Work?
Isotype controls work by acting as a benchmark for measuring background staining in immunological assays. The working mechanism of isotype control antibodies can be understood from the following aspects:
Detection of non-specific binding: Isotype control can help to identify and present the non-specific binding of antibodies. For example, the constant Fc region of an antibody may bind non-specifically to the Fc receptors on the cell surface. Isotype control antibodies can be used to distinguish the background of such non-specific binding.
Specificity verification: If the primary antibody binds to the target antigen and produces a signal in the experimental group, while the isotype control antibody does not produce a similar signal under the same experimental conditions, the specificity of the primary antibody can be confirmed. This comparison helps to accurately assess the reliability and accuracy of the experimental results.
Reducing the false positive rate: False positive results may be caused by non-specific binding or experimental conditions. By using isotype control antibodies, researchers can better control false positive results, thereby improving the overall quality of the experiment.
5. How to Choose An Appropriate Isotype Control Antibody?
Choosing the correct isotype control is a critical step to ensure that your experimental results are valid. Here are some important factors to consider when selecting an isotype control antibody:
5.1 Match the Host Species and Isotype of the Primary Antibody
The isotype control antibody should be raised from the same host species as the primary antibody. It also should match the subclass and light chain type of the primary antibody used in the experiment. This ensures that both antibodies will interact similarly with cells and tissues and is essential to minimize the risk of non-specific interactions caused by different antibody classes. If the primary antibody is an IgG1 from a mouse, then the isotype control must also be a mouse IgG1 antibody.
5.2 Verify the Specificity of the Isotype Control
Ensure that the selected isotype control antibody does not bind to any antigen in your specific experimental context, thereby reducing interference from the background signal.
5.3 Use the Same Concentration or Dose as the Primary Antibody
For consistency, the concentration or dose of the isotype control should be identical to that of the primary antibody. The isotype control treated group should be run under the same conditions as the experimental group. This ensures that any differences in binding are not due to variations in antibody concentration.
5.4 Use Conjugated or Unconjugated Isotype Controls
If your primary antibody is conjugated, choose a conjugated isotype control. If your primary antibody is unconjugated, an unconjugated isotype control is more appropriate.
6. Which Applications Use Isotype Control?
Isotype control plays an integral role in immunoassay techniques. They help researchers distinguish specific signals from background noise by providing a baseline, ensuring the accuracy and reliability of experimental results.
The use of isotype control antibodies in flow cytometry can help determine the specificity of cell surface markers. By using isotype controls in conjunction with the target antibody, researchers can clearly distinguish between true positive cells and false positive cells that appear due to non-specific binding. This is particularly important when analyzing cell populations to ensure the accuracy and reliability of the results.
In IHC experiments, the use of isotype controls can help eliminate background staining caused by nonspecific binding. By comparing the staining results of the experimental group and the isotype control group, researchers can more clearly identify true positive signals. Isotype controls provide necessary contrast in the process of labeling and quantifying specific tissues or cells.
In Western blotting, the use of isotype controls can help verify the specificity of the target protein. By comparing the signal of the target antibody and the isotype control, researchers can confirm that the observed signal is indeed the target protein and not other interfering substances.
In immunofluorescence microscopy, isotype controls help reduce the potential for non-specific fluorescence, ensuring that observed signals are truly due to antibody-antigen-specific interactions and not background fluorescence.
In ELISA, isotype control antibodies are used to evaluate the specificity of antibody binding and ensure that the observed signal is indeed due to the binding of the target primary antibody rather than other background factors. The use of isotype controls can help researchers determine the baseline of negative signals and adjust experimental conditions accordingly to improve the reliability of results.
6.6 Monoclonal Antibody Drug Studies
Isotope controls play an important role in the study of the efficacy and mechanism of action of monoclonal antibody drugs. Isotype control can serve as a baseline reference to evaluate the difference between the actual effect of antibody drugs on the target antigen and the non-specific effect, ensuring the validity and accuracy of the research results.
Conclusion
In immunological assays, using an isotype control antibody is not just a technical formality; it is a crucial step that can drastically improve the reliability and interpretability of your experimental results. By providing a "background" signal, the isotype control helps establish a threshold for acceptable non-specific binding in the experiment. This allows researchers to confidently interpret data and avoid overestimating the specific binding signal.
References
[1] Andersen MN, Al-Karradi SN, Kragstrup TW, Hokland M. Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages [J]. Cytometry A. 2016 Nov;89(11):1001-1009.
CUSABIO team. How to Choose An Appropriate Isotype Control Antibody?. https://www.cusabio.com/c-21207.html



Comments
Leave a Comment