Recombinant proteins play a critical role in modern biological research, with increasingly extensive applications ranging from basic studies and disease modeling to drug research and development. The ability to produce and manipulate proteins through recombinant DNA technology has revolutionized how researchers approach various biomedical challenges.
However, many researchers are perplexed about choosing the right recombinant protein for their experimental models. Selecting the appropriate recombinant protein for the specific experiment is vital for achieving reliable and reproducible results. This article aims to guide researchers through the key considerations in choosing the most suitable recombinant protein for their experimental model.
Table of Contents
1. What Are Recombinant Proteins?
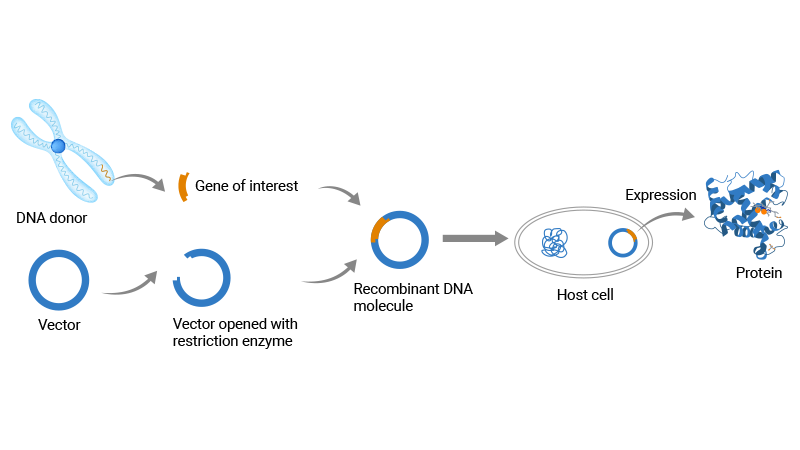
Recombinant proteins are proteins synthesized in the laboratory through genetic engineering technology, rather than being extracted directly from the organism. Recombinant proteins are produced by cloning a gene of interest into a vector, followed by expression in a host system (such as bacteria, yeast, or mammalian cells) using the cell's biosynthetic machinery. Recombinant proteins are essential reagents in areas like gene function studies, therapeutic development, and the production of biologics.
Figure 1. Schematic diagram of the production process of recombinant protein
2. Why Do We Need Recombinant Proteins?
Recombinant proteins are widely applied in various fields. Recombinant proteins are essential for advancing biomedical research, therapeutic development, and industrial applications. They enable precise study of protein interactions, disease mechanisms, and cellular responses through tools like ELISA, Western blot, and enzymatic assays.
In medicine, they replace defective proteins or provide targeted therapies for conditions like diabetes, cancer, and autoimmune disorders, offering safer, more specific treatments than small-molecule drugs. Their production in customizable systems (bacteria, yeast, or mammalian cells) ensures scalability and post-translational modifications critical for functionality. Beyond healthcare, recombinant proteins enhance agriculture (nutrient-rich animal feed) and food production (engineered enzymes for fermentation).
By combining scientific innovation with industrial efficiency, recombinant proteins drive breakthroughs in biotechnology and improve global health outcomes.
3. How to Choose the Right Recombinant Protein for Your Experimental Model?
Choosing the right recombinant protein for a specific experimental model requires considering multiple factors that directly influence the outcome of an experiment.
3.1 Define the Experiment Objective
The specific purpose of the experiment directly determines the selection criteria of the recombinant protein. Is the protein used for structural biology research, functional analysis, drug screening, or as an antigen for antibody production? Different research goals require different requirements for protein purity, activity, concentration, stability, and folding state.
Proteins used in cell culture research should be of high purity and biological activity to promote the desired cell outcomes. For instance, active cytokines are utilized to stimulate cell growth or differentiation. Growth factors should be chosen based on their effectiveness and stability in the culture medium.
When using recombinant proteins in clinical samples or animal models, protein stability, half-life, and bioactivity need to be taken into consideration. Delivery route (i.e., injection, local delivery) and potential immunogenicity of the protein are also factors.
The selection of recombinant proteins for targeting particular cell types, e.g., cancer cells, immune cells, or neuronal cells, would depend on their capacity to bind to the particular receptor or signaling pathway. In certain instances, targeted fusion proteins or antibody-drug conjugates (ADCs) can be employed.
Structural studies such as X-ray crystallography demand recombinant proteins with high purity (>95%) and correct folding. Functional studies, such as enzyme activity, use recombinant proteins with established biological activity. Tagged recombinant proteins (e.g., His, FLAG) are necessary for purification or detection in interaction studies such as Co-IP. Therapeutic recombinant proteins must be of GMP grade and low endotoxin.
3.2 Select the Appropriate Expression System
The expression systems offer the site and substrates required for the expression of recombinant DNA. The expression system directly impacts protein yield, protein folding, functionality, post-translational modifications (PTMs), and bioactivity.
Common expression systems include bacteria (such as E. coli), yeast, baculovirus-infected insect cells, mammalian cells, and in vitro E. coli (cell-free). Each expression system has varying advantages and limitations [1].
| Expression System |
Advantages |
Disadvantages |
Suitable recombinant protein type |
Most Common Application |
| Prokaryotic expression system (E. coli) |
- Inexpensive fermentation requirements [2,3]
- Rapid proliferation ability [2,3]
- Stable high-level expression
- Easily achieve high cell density cultures [4,5]
- Well-known biochemistry and genetics [2,3]
|
- Lacks eukaryotic post-translational modifications (PTMs)
- Inclusion body (IB) formation: Lack of eukaryotic protein folding machinery, leading to inclusion body formation [6]
- Codon bias
- Endotoxin issues
|
- Small molecular weight protein (<100 kDa)
- Proteins that do not require PTMs
|
- Structural studies
- Enzymes/antigens without PTM requirements
|
| Yeast (e.g., Pichia pastoris) |
- Performs certain PTMs such as N or O-linked glycosylation, acetylation, amidation, hydroxylation, methylation, phosphorylation, pyrrolidone carboxylic acid, sulfation, and ubiquitylation [7]
- Scalable up to high-density fermentation for laboratory production
- Higher protein yields
- Efficient protein folding and secretory expression
- Easy genetic manipulation
- Available complete genome sequences
|
- Limited to yeast-specific PTMs: not suitable for proteins requiring complex human-like PTMs
- Complex culture media conditions
- Challenging for high molecular weight proteins
|
- Proteins that require glycosylation or other simple PTMs
- Secretory proteins
|
- Secreted proteins
- Industrial enzymes
- Vaccine antigens
|
| Baculovirus expression in insect cells [8] |
- Supports complex PTMs, similar to mammalian protein processing
- High yield for large, complex proteins and virus-like particles (VLPs)
- Enables correct folding and activity for most proteins
- High safety: baculovirus is not infectious to mammals
|
- Medium culture cycle: slower than bacterial/yeast systems
- Viral vector dependency increases complexity
- Glycosylation differs from mammalian systems (e.g., lacks sialic acid)
- Producing recombinant baculovirus vectors is time-consuming
|
- Membrane proteins
- Viral vaccines [9]
|
- Membrane proteins
- Viral-like particles (VLPs)
- GPCRs
- Secreted growth factors
|
| Mammalian Cells (HEK/CHO)[10] |
- Performs native-like PTMs (humanized glycosylation pattern)
- High-fidelity folding and secretion
- Compatibility with human proteins
- Suitable for expressing proteins with complex structures, such as antibodies and membrane proteins
|
- High cost
- Slow growth: long production time, low production efficiency
- Complicated technology
- High risk of contamination (virus, prions, oncogenic DNA)
- Very low scale-up capacity
|
- Biopharmaceutical proteins [11,12]
- Proteins requiring native-like PTMs
|
- Monoclonal antibodies
- Complex glycoproteins
- Clinical/therapeutic proteins
|
| Cell-Free (In Vitro E. coli )[13] |
- Rapid and efficient expression: protein can be obtained within hours without cell culture
- Bypasses cell viability constraints
- Flexible for toxic proteins or proteins difficult to express within cells
- Easy isotopic labeling
|
- Very high cost per mg
- Limited scalability
- Short protein half-life
- Limited to PTMs
|
- Toxic proteins
- Small molecular weight protein
|
- High-throughput screening
- Toxic proteins
- Synthetic biology applications
|
CUSABIO can provide five expression systems for recombinant protein production, including:
Recombinant protein expression in E. coli
Recombinant protein expression in yeast
Recombinant protein expression in insect-Baculovirus
Recombinant protein expression in mammalian cells
Recombinant protein expression in vitro E. coli
3.3 Addition of Protein Tags
Many recombinant proteins are co-expressed with protein tags (e.g., His-tag, GST-tag, MBP-tag, and FLAG-tag) to facilitate protein detection, purification, and solubility. These protein tags are added to the protein either at the N- or C-terminus and are generally small peptides that can be detected by specific antibodies or other binding molecules.
The selection of a tag can influence the solubility, stability, and bioactivity of the protein. Additionally, tags can interfere with protein function and must be removed if required.
3.4 Recombinant Protein Purification and Quality Control
There are various purification methods for recombinant proteins, and each method affects the expression level, activity, conformation, purity, and stability of proteins differently. Affinity chromatography and ion-exchange chromatography are the most frequently used techniques; the former provides protein separation by specific interactions between tags (e.g., His tag, GST tag, MBP tag, etc.) and corresponding mediators, while the latter utilizes the charge nature of proteins for effective separation.
Affinity chromatography generally achieves high purity in protein isolation, yet some minor impurities might still be present. Some tags may influence the natural confirmation or function of the protein and require removal post-purification to reinstate activity. Ion exchange chromatography is sensitive to the charge distribution of proteins and can achieve high-resolution separation. However, extreme pH conditions may cause protein denaturation and necessitate the optimization of buffering conditions.
Quality control is essential, given that even trace impurities or improperly folded proteins can influence experimental outcomes. Techniques including SDS-PAGE, Western blotting, and mass spectrometry are frequently utilized to evaluate protein purity, identity, and post-translational modifications. Furthermore, endotoxin level testing by the LAL assay should be conducted to guarantee the safety of proteins for in vivo applications.
3.5 Functional Validation of Recombinant Proteins
Upon purification, recombinant proteins must go through functional validation to guarantee they exhibit the desired biological activity. This is achieved through in vitro or in vivo experiments, including enzyme activity assays, ELISA, cell proliferation assays, receptor-ligand binding assays, or animal model experiments.
3.6 Balancing Cost and Time Efficiency
When choosing a recombinant protein, cost and time also need to be taken into consideration. For instance, while the prokaryotic system is cost-effective and rapid, it may not satisfy the stringent requirements for protein quality. Conversely, the eukaryotic system, while expensive and time-consuming, may yield higher-quality proteins. Consequently, researchers must select the appropriate expression system based on the specific experimental needs, such as protein complexity, budget constraints, and time limitations.
Scientists can optimize the experimental process by evaluating the merits and drawbacks of various expression systems, thus attaining high-quality protein with budgetary and time limitations. This strategy improves the effectiveness of experiments and facilitates straightforward follow-up research activities.
It is advisable to prioritize high-quality recombinant proteins produced by mammalian systems if the budget permits. When there are severe time limitations for experimentation, commercially available proteins are usually chosen. When expressing autonomously, both the purification equipment and technical demands must be taken into account.
3.7 Choose A Reliable Supplier of Recombinant Proteins
Reliable suppliers typically possess a comprehensive production and quality control system, enabling them to provide products with consistent performance to ensure the reproducibility of experiments and the precision of outcomes. They use the latest technology along with expert know-how to enhance the manufacturing process so that the proteins maintain high purity and functionality levels.
Moreover, they adhere to rigorous virus safety assessment procedures and provide detailed assessment reports to guarantee the products are viral contaminant-free.
Additionally, suppliers provide detailed product information, encompassing aspects such as sequence, purity, and activity, along with validated experimental data or references to aid researchers in assessing the suitability of proteins and minimizing experimental uncertainties. A guaranteed supply chain coupled with an improved after-sales service ensures that products are delivered on time while effectively resolving hurdles during use.
4. Future Trends and Innovations
Recent innovations in recombinant protein technology include the application of artificial intelligence (AI) for protein structure prediction and design. In addition, new expression systems like cell-free synthesis and the use of CRISPR-based techniques for optimizing protein expression in mammalian cells are expected to enhance protein production efficiency. Advances in high-throughput purification techniques also promise to reduce time and cost.
Conclusion
Selecting the right recombinant protein requires careful consideration of multiple factors, including the experimental objective, expression system, protein tags, protein purification and quality, protein validation, and protein supplier. Researchers must match their protein choices to the specific needs of their experimental model, ensuring that the protein is pure, active, and stable. Ongoing advancements in recombinant protein technology will continue to provide new tools and strategies to streamline the selection and production of recombinant proteins, ultimately enhancing experimental outcomes.
References
[1] Tripathi, N. K., & Shrivastava, A. (2019). Recent Developments in Bioprocessing of Recombinant Proteins: Expression Hosts and Process Development [J]. Frontiers in Bioengineering and Biotechnology, 7, 420.
[2] Baeshen M. N., Al-Hejin A. M., et al. (2015). Production of biopharmaceuticals in E. coli: current scenario and future perspectives [J]. J. Microbiol. Biotechnol. 25, 953–962.
[3] Gupta S. K., Shukla P. (2016). Advanced technologies for improved expression of recombinant proteins in bacteria: perspectives and applications [J]. Crit. Rev. Biotechnol. 36, 1089–1098.
[4] Lee S. Y. (1996). High cell-density culture of Escherichia coli [J]. Trends Biotechnol. 14 98–105.
[5] Shiloach J., Fass R. (2005). Growing E. coli to high cell density – a historical perspective on method development [J]. Biotechnol. Adv. 23 345–357.
[6] Baig, F., Fernando, L. P., Salazar, M. A., Powell, R. R., Bruce, T. F., and Harcum, S. W. (2014). Dynamic transcriptional response of Escherichia coli to inclusion body formation [J]. Biotechnol. Bioeng. 111, 980–999.
[7] Baghban R., Farajnia S., Rajabibazl M., Ghasemi Y., Mafi A., Hoseinpoor R., Rahbarnia L., Aria M. Yeast Expression Systems: Overview and Recent Advances [J]. Mol. Biotechnol. 2019;61:365–384.
[8] Contreras-Gómez A., Sánchez-Mirón A., García-Camacho F., Molina-Grima E., Chisti Y. (2014). Protein production using the baculovirus-insect cell expression system [J]. Biotechnol. Prog. 30, 1–18.
[9] Felberbaum R. S. (2015). The baculovirus expression vector system: A commercial manufacturing platform for viral vaccines and gene therapy vectors [J]. Biotechnol. J. 10, 702–714.
[10] Michael I.P., Nagy A. In: Recombinant Protein Expression in Mammalian Cells Methods in Molecular Biology. Hacker D.L., editor. Springer New York; 2018. Inducible Protein Production in 293 Cells Using the piggyBac Transposon System [J]. pp. 57–68.
[11] Butler M (2005) Animal cell cultures: recent achievements and perspectives in the production of biopharmaceuticals [J]. Appl Microbiol Biotechnol 68: 283–291.
[12] Zhu J (2012) Mammalian cell protein expression for biopharmaceutical production [J]. Biotechnol Advan 30: 1158–1170.
[13] Foshag D., Henrich E., Hiller E., Schäfer M., Kerger C., Burger-Kentischer A., Diaz-Moreno I., García-Mauriño S.M., Dötsch V., Rupp S., Bernhard F. The E. coli S30 lysate proteome: A prototype for cell-free protein production [J]. N. Biotech. 2018;40:245–260.
CUSABIO team. How to Choose the Right Recombinant Protein for Your Experimental Model?. https://www.cusabio.com/c-21219.html




Comments
Leave a Comment