The landscape of therapeutic antibody discovery has undergone a revolutionary transformation since Emil von Behring's antitoxin concept (1890) [1] and the seminal hybridoma technology by Köhler and Milstein (1975) [2]. While phage display (1990) [3] enabled in vitro library generation, it often compromised native antibody pairing. Single B cell platforms now represent the vanguard of antibody discovery, enabling direct interrogation of antigen-specific B lymphocytes while preserving natural heavy-light chain pairing and post-translational modifications. This technology accelerates the isolation of diverse, high-affinity antibodies against complex targets—from oncology biomarkers to multi-subunit receptors—with unprecedented speed and precision.
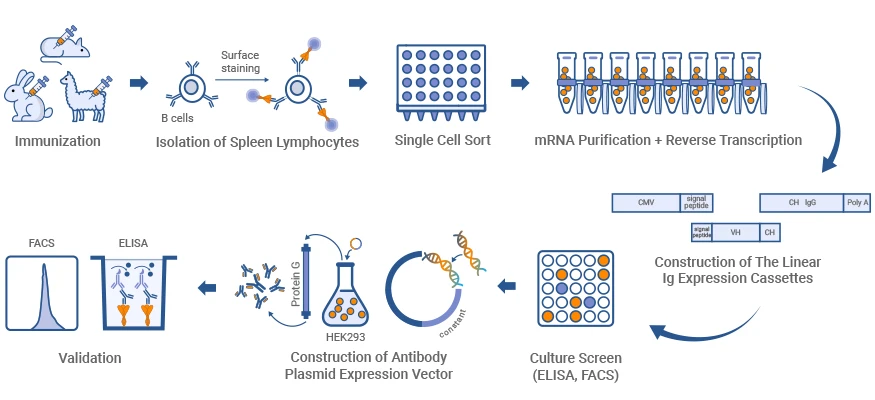
1. What Is Single B Cell Antibody Technology?
Single B cell antibody technology is an advanced methodology that involves isolating antigen-specific B cells, recovering paired heavy (VH) and light (VL) chain sequences, and producing monoclonal antibodies (mAbs) from individual lymphocytes. This approach shortens development timelines, maintains natural VH-VL pairing, and supports high-throughput discovery compared to bulk methods or display technologies.
Figure 1. Diagram of Single B cell sorting and antibody generation
2. Advantages of Single B Cell Technologies for Monoclonal Antibody Discovery
Compared to hybridoma technology, signal B cell discovery technology has several advantages, including short-cycle development, high efficiency, and high throughput. For antibody display technology, signal B cell technology is featured with natural VHand VL pairing, native post-translational modification profile, and broad diversity. Signal B cell discovery technology is easy to manipulate, low-cost, and high-accuracy compared to proteomics technology.
Single B cell antibody discovery technology excels in the discovery and characterization of highly specific antibodies, which are ideal antibody candidates for antibody-drug conjugates (ADCs).
3. Case Study: TROP2 Monoclonal Antibody Development
TROP2 is a surface glycoprotein highly expressed in many epithelial cancers, including breast, lung, and gastrointestinal tumors, but with limited expression in normal tissues [5]. It promotes cancer progression and metastasis by enhancing cell growth, migration, and survival. Due to its role in tumor aggressiveness and poor prognosis, TROP2 has become a promising molecular target for targeted therapy like antibody-drug conjugates. Therefore, it is crucial to develop high-quality TROP2 monoclonal antibodies.
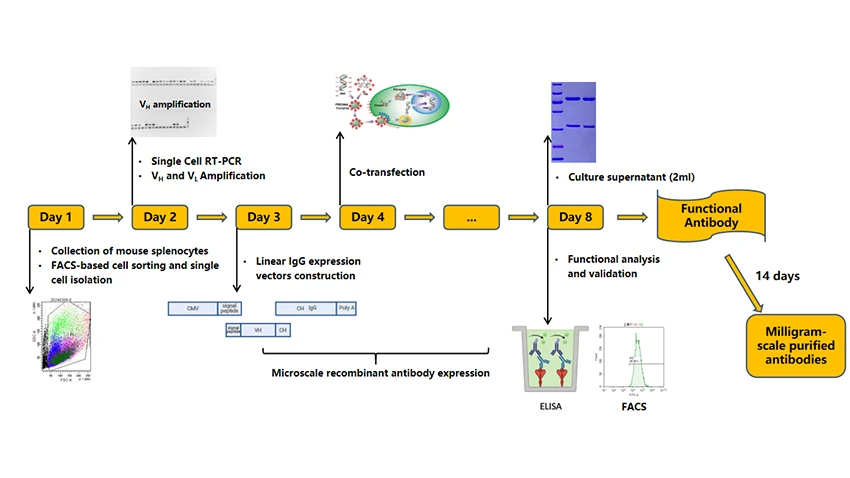
Workflow of TROP2 Monoclonal Antibody Production via Single B Cell Antibody Technology
CUSABIO can produce functional TROP2 monoclonal antibodies in small-scale supernatant (2mL) as quickly as 8 days using single B cell antibody technologies. Milligram-scale purified TROP2 monoclonal antibodies can be obtained within 22 days through the same process.
Figure 2. Workflow of TROP2 monoclonal antibody production via single B cell antibody technology
Day 1: B Cell Sorting and Isolation
- Collection of Mouse Splenocytes: Splenocytes are extracted from a mouse that has been immunized with recombinant human TROP2 protein. These cells contain B cells that produce antibodies against the TROP2 protein.
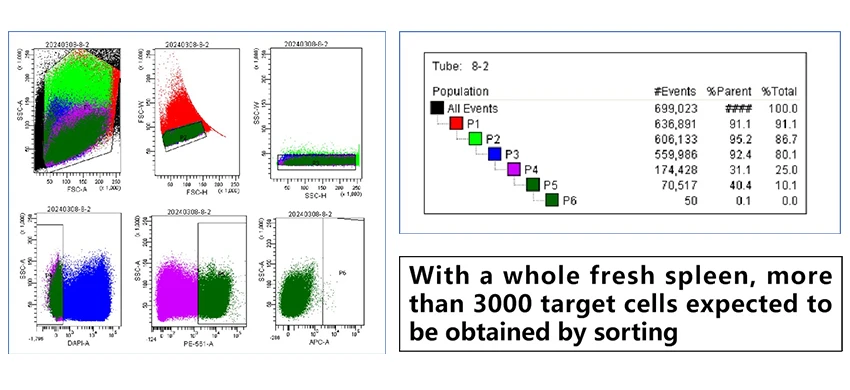
- FACS-Based Cell Sorting and Single B Cell Isolation: The collected splenocytes are sorted using FACS to isolate individual B cells. Each B cell that produces an antibody against TROP2 is isolated into a single well, ensuring that each well contains a unique B cell for further analysis and characterization.
Figure 3. FACS-based B cell sorting
Day 2: VH and VL Amplification
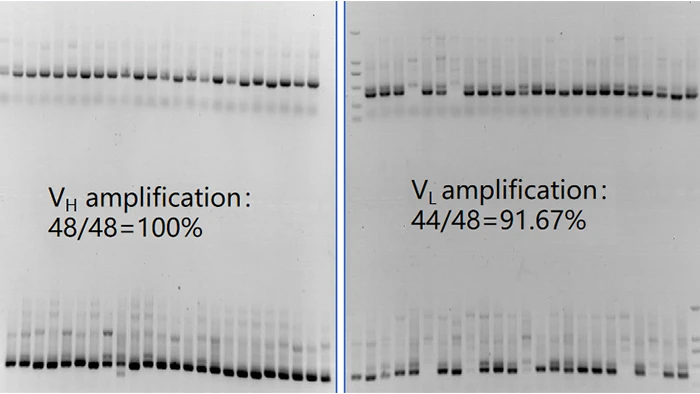
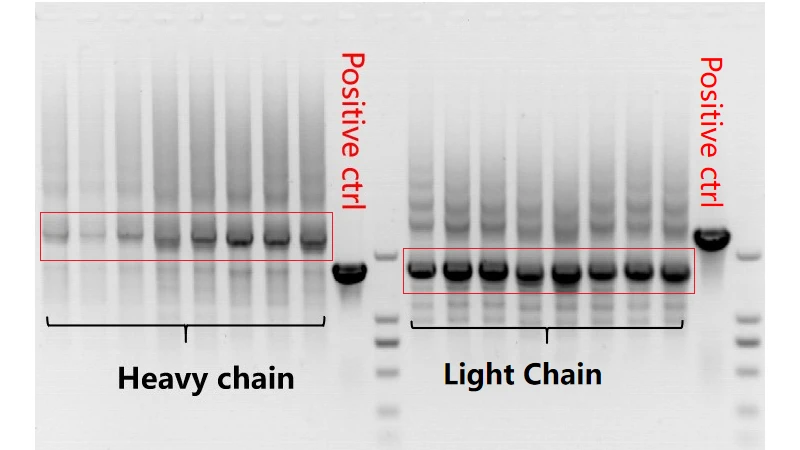
- Single-Cell RT-PCR: After sorting, each single B cell is lysed to extract mRNA, which is further reverse-transcribed into cDNA. The cDNA is used as a template for PCR amplification of the variable regions of the heavy chain (VH) and light chain (VL) of the antibody genes.
Figure 4. VH and VL amplification SDS-PAGE
This electrophoretic analysis validates the exceptional amplification efficiency and robust stability of the CUSABIO single B-cell antibody discovery platform throughout the PCR amplification process.
Day 3: Linear IgG Expression Vectors Construction
- The CDR3 sequences are extracted from amplified VH and VL and sequenced to assess the diversity of TROP2 antibodies.
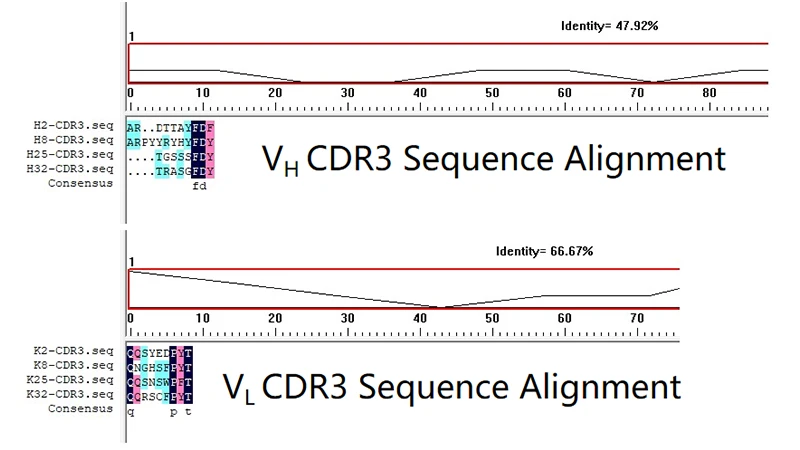
Figure 5. Sequencing and diversity assessment of VH and VL sequences
This figure shows that the identity of the VH CDR3 sequences is only 47.92%, indicating that the VH CDR3 of these four antibody clones (H2, H8, H25, H32) have significant differences in the amino acid sequence and are highly diverse. CDR3, especially the VH CDR3, is the most core and contributing region when the antibody binds to the antigen. The identity of the VL CDR3 sequence is 66.67%, which is significantly higher than that of the heavy chain, indicating that the VL CDR3 sequence is relatively conserved.
CUSABIO has successfully obtained a set of TROP2 monoclonal antibodies with high diversity, especially in the heavy chain. CUSABIO can further select VH-VL pairs from these amplified genes.
- The recovered, paired VH/VL is ligated with regulatory elements to construct linear IgG expression vectors.
Figure 6. VH and VL genes SDS-PAGE diagram
This figure shows that the electrophoretic bands are clear and fall within the expected size range (the specific lengths of the VH and VL genes), indicating that the VH/VL genes have been successfully cloned into the linear expression vectors.
Day 4: Co-Transfection
- The constructed linear expression vectors are transfected into HEK 293 cells for antibody production.
Day 5-8: Culture of Functional Antibodies
- The transfected cells are cultured in appropriate conditions for 4 days.
- After this period, the functional antibodies are harvested from the culture supernatant (2ml) and subjected to functional analysis and validation.
Functional Analysis and Validation
Functional analysis is carried out using ELISA and FACS to confirm the binding specificity and functionality of the antibodies.
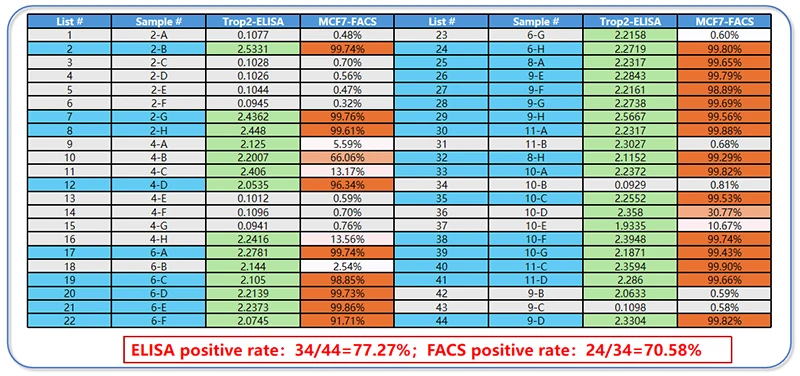
Figure 7. ELISA and FACS detection data table
The ELISA assay, which measures direct binding to the recombinant TROP2 antigen, identified 34 out of 44 samples (77.27%) as positive. This indicates a high success rate in the initial screening phase, confirming that the majority of the antibodies generated by the single B cell technology are expressible and possess inherent binding capability to TROP2.
A subsequent, more stringent functional assay was performed using FACS analysis on MCF7 cells. From the 34 ELISA-positive clones, 24 (70.58%) were confirmed to be positive. This demonstrates that a significant proportion of the antibodies can bind to the native, conformationally intact TROP2 protein presented on the surface of living cells.
15-22 Days: Milligram-Scale Purified Antibodies
- After successful characterization and validation, the TROP2 monoclonal antibody is scaled up for larger production. Milligram-scale purified antibodies are obtained, and the entire process, from cell sorting to antibody production, can be completed in 22 days.
In summary, CUSABIO can produce functional TROP2 monoclonal antibodies in small-scale (2 mL) culture supernatant in as few as 8 days, and deliver milligram quantities of purified antibody within 22 days.
4. Case Study 2: ITGAV & ITGB6 Antibody Development
Increased integrin αvβ6 expression and aberrant TGF-β1 activation and function are associated with organ fibrosis and cancer. Therefore, integrin αvβ6 is an attractive target for both cancer imaging and the treatment of fibrosis and cancer. Its conformational complexity challenges conventional discovery platforms.
Integrated Experimental Flow and Result Analysis
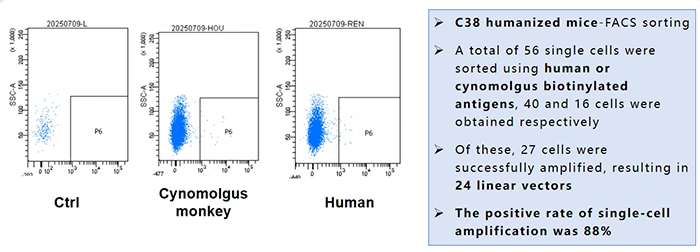
① Single B Cell Screening-FACS
- Objective: To screen for B cells isolated from fully humanized C38 mice that are capable of binding human or cynomolgus monkey (cyno)-derived ITGAV & ITGB6 antigens.
- Key Results:
- 56 single cells were sorted (40 binding human antigen, 16 binding cyno antigen).
- 27 expansion-positive cell cultures were successfully obtained, leading to 24 linear expression vectors.
- The positive rate for single-cell expansion reached 88%, indicating excellent screening efficiency.
- Conclusion: Parallel screening using both human and cynomolgus monkey antigens allows for the simultaneous identification of antibodies with cross-reactivity to both species. This is crucial for preclinical studies, where safety and efficacy need to be validated in non-human primates.
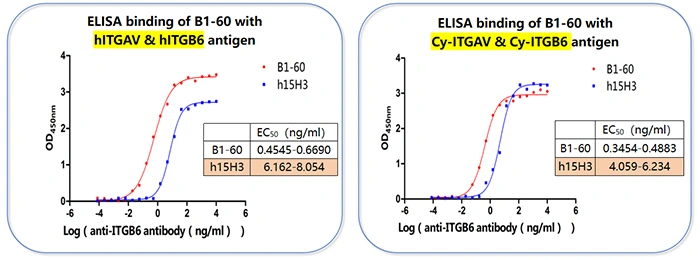
② Binding Activity Validation (ELISA Analysis)
- Objective: To validate the binding characteristics of the antibodies to antigens in different formats.
- Key Findings:
- Conclusion: EC₅₀ values at the sub-nanogram/milliliter level indicate that B1-60 possesses extremely high antigen-binding affinity. Its maintained cross-reactivity with both human and cyno antigens predicts good species adaptability, which is valuable for subsequent development stages.
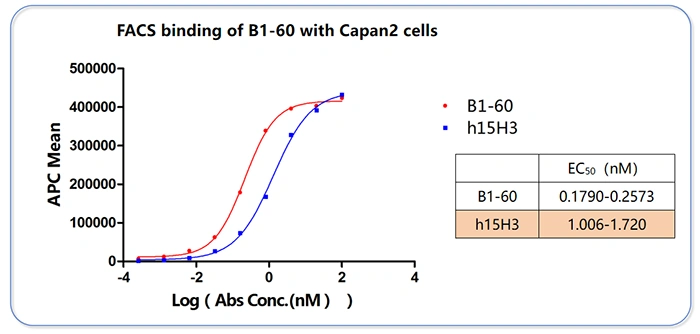
③ Functional Activity Validation (FACS-based Cell Binding Assay)
- Objective: To validate antibody function at the cellular level, which is closer to a physiological state.
- Key Findings:
- Used Capan-2 cells (a pancreatic cancer cell line) that natively express the ITGAV/ITGB6 heterodimer.
- B1-60 exhibited potent cell-binding capability: EC₅₀ = 0.1790-0.2573 nM.
- Compared to the control antibody h15H3 (EC₅₀ = 1.006-1.720 nM), the activity of B1-60 was approximately 5 to 10 times higher.
- Conclusion: B1-60 effectively recognizes the physiological conformation of the target on live cells, confirming functional relevance. This is a key piece of evidence for the antibody's functionality.
The data strongly support B1-60 as a high-value antibody with excellent binding, cross-species reactivity, and functional activity against the ITGAV/ITGB6 target.
In summary, single B cell platforms have revolutionized antibody discovery by merging high-throughput screening with native biological fidelity. These technologies overcome the limitations of hybridoma and phage display, enabling rapid isolation of diverse, high-affinity antibodies against challenging targets like membrane proteins and multi-subunit complexes. The power of single B cell technology lies in its ability to harness the full breadth of the immune repertoire, transforming antibody discovery into a precision engine for biological innovation.
CUSABIO's single B cell platform utilizes an efficient two-round biomarker sorting strategy to precisely isolate antigen-specific B cells. A key innovation is the introduction of linear vector construction combined with a PCR assembly method, which significantly shortens the timeline and enhances overall efficiency. Case studies targeting TROP2, αvβ6 integrin, and emerging oncology antigens underscore the platform’s versatility across therapeutic areas. The platform's robustness is demonstrated by its track record, having successfully completed over 10 projects with a 100% delivery rate for high-value targets such as Trop2, TM4SF1, BCMA, ROR1, and ADAM9.
References
[1] Behring E, Kitasato S. 1890. Über das Zustandekommen der Diphtherie-Immunität und der Tetanus-Immunität bei Thieren [J]. Dtsch Med Wochenschrift 49:1113–1114.
[2] KÖHLER, G., & MILSTEIN, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity [J]. Nature, 256(5517), 495-497.
[3] McCafferty, J., Griffiths, A. D., Winter, G., & Chiswell, D. J. (1990). Phage antibodies: Filamentous phage displaying antibody variable domains [J]. Nature, 348(6301), 552-554.
[4] Schardt, J.S., Sivaneri, N.S. & Tessier, P.M. Monoclonal Antibody Generation Using Single B Cell Screening for Treating Infectious Diseases [J]. BioDrugs 38, 477–486 (2024).
[5] Pandit, B. R., Unakal, C., Vuma, S., & Akpaka, P. E. (2025). A Comprehensive Review About the Use of Monoclonal Antibodies in Cancer Therapy [J]. Antibodies, 14(2), 35.
CUSABIO team. Accelerating Diverse Antibody Discovery: The Power of Single B Cell Platforms. https://www.cusabio.com/c-21259.html




Comments
Leave a Comment