Call us
301-363-4651 (Available 9 a.m. to 5 p.m. CST from Monday to Friday)
| Code | CSB-EL013066BO |
| Size | 96T,5×96T,10×96T |
| Price | Request a Quote |
| Trial Size |
24T ELISA Kit Trial Size (Only USD$150/ kit) * Sample kit cost can be deducted as a $30 credit for each 96-assay kit of the same analyte and brand you subsequently purchase within six months until depleted. More details >> Interested in a trial size? Please leave a message below.
|
| Have Questions? | Leave a Message or Start an on-line Chat |
| Intra-assay Precision (Precision within an assay): CV%<8% | |||||||
| Three samples of known concentration were tested twenty times on one plate to assess. | |||||||
| Inter-assay Precision (Precision between assays): CV%<10% | |||||||
| Three samples of known concentration were tested in twenty assays to assess. | |||||||
| These standard curves are provided for demonstration only. A standard curve should be generated for each set of samples assayed. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
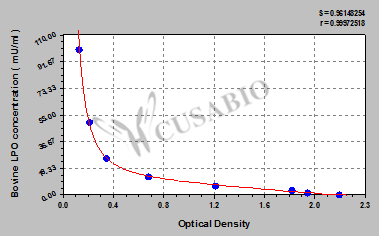
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This Bovine LPO ELISA Kit was designed for the quantitative measurement of Bovine LPO protein in breast milk. It is a Competitive ELISA kit, its detection range is 1.56 mU/mL-100 mU/mL and the sensitivity is 1.56 mU/mL.
There are currently no reviews for this product.