Parathyroid Hormone 1 Receptor (PTH1R), a key member of the Class B G protein-coupled receptor (GPCR) family, is widely distributed in various tissues such as bones, kidneys, brain, and heart. As a central regulator of calcium-phosphorus homeostasis and bone metabolism, PTH1R binds to parathyroid hormone (PTH) and parathyroid hormone-related peptide (PTHrP), activating multiple downstream signaling pathways (e.g., Gs/cAMP/PKA, Gq/PLC/PKC), and participating in embryonic development, calcium-phosphorus metabolism, tumor progression, and the pathophysiology of multiple diseases. Its dysfunction is closely associated with osteoporosis, hypoparathyroidism, primary failure of tooth eruption (PFE), and other disorders, making it an important target for drug development.
1. PTH1R Structure and Regulatory Mechanisms
1.1 Key Structural Domain Analysis
● N-terminal Extracellular Domain (ECD)
The large N-terminal ECD of PTH1R is responsible for recognizing and binding ligands (PTH or PTHrP). This domain plays a critical role in initial ligand binding, featuring high flexibility and specificity.
● 7-transmembrane Domain (7TMD)
Composed of seven transmembrane α-helices (TM1-TM7) and connecting loops, it transmits hormone-binding signals and interacts with G proteins. Conformational changes in the transmembrane domain are central to receptor activation, triggering downstream signal transduction.
● C-terminal Intracellular Domain
Additionally, the transmembrane helices and connecting loops of PTH1R are crucial for ligand-induced receptor activation, while the coordination between ECD and the transmembrane domain ensures high-affinity ligand binding and precise signal transmission.
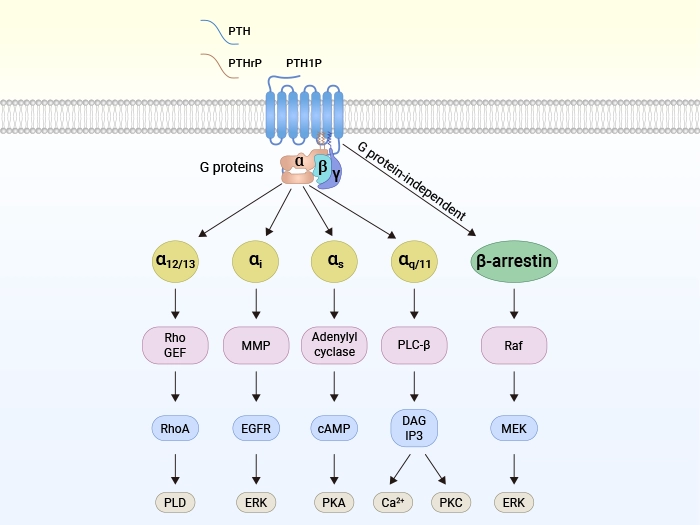
1.2 Signal Regulation
Upon activation by ligands (e.g., PTHrP), PTH1R mediates physiological effects through multiple signaling pathways:
Classical G Protein-dependent Pathways
● Gαs/cAMP/PKA pathway: Activates adenylate cyclase (AC) to generate cAMP, which activates protein kinase A (PKA), regulating osteoblast differentiation and calcium-phosphorus metabolism.
● Gαq/PLC/PKC pathway: Generates diacylglycerol (DAG) and inositol trisphosphate (IP3) via phospholipase C (PLC), activating protein kinase C (PKC), primarily regulating renal functions.
● Gα12/13/RhoA/PLD pathway: Regulates cytoskeletal reorganization and intracellular lipid metabolism, influencing cell morphology and function.
β-arrestin Non-classical Pathway:
After PTH1R binds with β-arrestin, it can activate the ERK-1/2/MAPK pathway, prolong cAMP signal duration, and promote extracellular signal responses. This pathway can be activated either through G protein-dependent pathways or through β-arrestin's direct activation independent of G proteins.
Specific Regulatory Mechanisms
The β-arrestin-dependent signaling of PTH1R exerts unique effects on bone metabolism, such as promoting osteoblastic anabolism through β-arrestin-mediated ERK activation. Notably, unlike other GPCRs (e.g., DP2, GPR17, FFA2), the β-arrestin pathway of PTH1R can efficiently activate ERK signaling even in the absence of functional G proteins, highlighting the diversity and flexibility of its signal transduction.
The synergistic effects of these signaling pathways make PTH1R a key regulatory target for bone metabolism, calcium homeostasis, and various pathological processes (e.g., osteoporosis, renal fibrosis).
Figure: Schematic of ligand binding to PTH1R and downstream signaling pathways [1]
2. PTH1R and Diseases
● PTH1R and Osteoporosis
PTH1R is the core regulatory switch for bone metabolism [2,3,4]. Its intermittent activation promotes osteoblast activity and inhibits sclerostin, primarily driving bone formation while mildly regulating bone resorption, thereby increasing bone mass to counteract osteoporosis. In contrast, its sustained activation significantly upregulates the RANKL/OPG ratio, strongly stimulating osteoclast-mediated bone resorption, leading to bone loss and causing or exacerbating osteoporosis. This difference in signal duration determines its ultimate effect on bone balance. Therefore, PTH1R is a core molecule for understanding bone metabolic balance, osteoporosis pathogenesis, and developing related targeted therapies.
● PTH1R and Intervertebral Disc Degeneration (IVDD)
PTH1R shows significant downregulation in intervertebral disc degeneration (IVDD), with its activity reduction positively correlating with degeneration severity in both human and animal models. Research [5] shows that low-concentration oxidative stress (such as 30 μM TBHP) can transiently upregulate PTH1R, promoting nucleus pulposus cells to synthesize key matrix components like type II collagen and Sox9 while inhibiting degrading enzymes (MMP-13/ADAMTS5), exerting compensatory protective effects. In contrast, high-concentration oxidative stress (75 μM TBHP) leads to sharp PTH1R decline, causing extracellular matrix metabolic imbalance and accelerating degeneration. This suggests PTH1R may be a potential intervention target for IVDD progression by regulating oxidative stress responses and ECM homeostasis.
● PTH1R and Diabetic Nephropathy
Diabetic nephropathy is a common complication of diabetes. Studies [6] have found that in streptozotocin (STZ)-induced diabetic mouse models, the expression of parathyroid hormone-related protein (PTHrP) and its receptor PTH1R in renal tissues is significantly upregulated. Hyperglycemia can promote PTHrP expression by activating the angiotensin II (Ang II) and its AT1 receptor pathway, and may upregulate PTH1R through non-AT1 receptor-dependent mechanisms. Additionally, PTHrP not only affects renal tubular epithelial cell proliferation and inflammatory responses through autocrine/paracrine mechanisms but may also exert intrinsic regulatory effects through nuclear localization, thereby participating in the development of glomerular hyperfiltration, renal hypertrophy, and proteinuria in diabetic nephropathy. Research indicates that abnormal activation of the PTHrP/PTH1R system is closely related to diabetic renal injury, suggesting it may serve as an important indicator for predicting early proteinuria and disease progression.
● PTH1R and Hypoparathyroidism
Hypoparathyroidism is a disease characterized by calcium-phosphorus metabolism disorders caused by insufficient parathyroid hormone secretion or functional defects. In its pathogenesis, the parathyroid hormone 1 receptor is a key regulatory point [7]. When PTH1R function is abnormal (such as receptor gene mutations, antibody blockade, or downstream signaling defects), parathyroid hormone cannot effectively activate this receptor and its downstream Gsα/cAMP/PKA signaling pathway, leading to failure in target organ responses such as reduced renal calcium reabsorption, obstructed phosphorus excretion, and insufficient bone calcium mobilization, ultimately causing characteristic hypocalcemia and hyperphosphatemia.
● PTH1R and Breast Cancer
As the common receptor for PTH and PTHrP, PTH1R plays a central regulatory role in breast cancer bone metastasis. Swami et al. [8] found that intermittent PTH treatment inhibits bone metastasis formation by activating the PTH1R signaling pathway in breast cancer cells and osteoblasts, with this effect dependent on PTH1R presence in target cells—when breast cancer cells or osteoblasts lack PTH1R, intermittent PTH treatment cannot exert anti-metastatic effects. PTH1R alters the bone-breast cancer vicious cycle by regulating bone microenvironment gene expression (e.g., upregulating Vcam1, Cxcl12, Flt1, etc.), while inhibiting the expression of pro-osteolytic factors like PTHrP, thereby weakening tumor cell tropism and colonization capacity in bone tissue. As a PTH1R ligand, PTHrP exhibits dual roles in early tumor progression inhibition and late bone metastasis promotion, ultimately demonstrating that the PTH1R-mediated signaling network is a key target for intervening in breast cancer bone metastasis.
3. Current Status of PTH1R Drug Development
3.1 Marketed Drugs
Currently, marketed drugs targeting PTH1R are primarily peptide hormone analogs, with indications focusing on osteoporosis and hypoparathyroidism. Representative drugs include:
Teriparatide: The first approved PTH1R agonist, a recombinant human PTH (1-34) fragment. It activates the Gαs/cAMP pathway via intermittent subcutaneous injection, significantly promoting osteoblast-mediated bone formation for treating severe osteoporosis. Despite efficacy, its short half-life, daily injection requirement, and potential risks of hypercalcemia and osteosarcoma limit long-term use.
Teriparatide (Forteo®)
(Image source: Eli Lilly and Company)
Abaloparatide: A selective PTH1R agonist with higher bias for Gαs signaling, reducing bone resorption-related side effects. Clinical data show a ~50% lower incidence of hypercalcemia, approved for postmenopausal osteoporosis.
3.2 Investigational Drugs
Long-acting PTH analogs: Such as MT1013, the world's first dual-target peptide agonist designed to treat secondary hyperparathyroidism (SHPT) through a dual mechanism with osteogenic growth peptide-like effects. It reduces PTH to baseline levels while decreasing hypocalcemia incidence and improving renal osteodystrophy and anemia conditions. Currently in Phase III clinical trials.
Hormone replacement therapy: Such as MBX 2109, a weekly parathyroid hormone peptide prodrug expected to become a long-acting hormone replacement therapy. It received orphan drug designation from the U.S. FDA in July 2022 for treating chronic hypoparathyroidism (HP) and is currently in Phase II clinical trials.
Overall, PTH1R-targeted drug development shows a diversified trend, with long-acting modifications focused on recombinant peptide hormones (e.g., PTH analogs), while small-molecule agonists and other new formulations are rapidly evolving.
| Drug |
Drug Type |
Indication |
Developing Institution |
Highest Phase |
| Teriparatide |
Peptide |
Osteoporosis |
Eli Lilly & Co. |
Marketed |
| Abaloparatide |
Peptide |
Osteoporosis |
Radius Health, Inc. |
Marketed |
| Natpar |
Hormone |
Hypoparathyroidism, hypocalcemia |
Shire Plc |
Marketed |
| Eneboparatide (AZP-3601) |
Peptide |
Hypoparathyroidism |
Amolyt Pharma |
Phase III |
| MT-1013 |
Peptide |
Secondary hyperparathyroidism, chronic kidney disease, hypercalcemia |
Shanxi Micot Technology Co., Ltd. |
Phase III |
| Recombinant Human PTH for Injection |
Hormone |
Osteoporosis |
Shanghai Celgen Bio-pharmaceutical Co., Ltd. |
Phase II |
| Canvuparatide (MBX-2109) |
Small molecule |
Hypoparathyroidism |
MBX Biosciences, Inc. |
Phase II |
| RT-102 |
Peptide |
Osteoporosis |
Rani Therapeutics LLC |
Phase I |
4. Future Prospects for PTH1R-targeted Therapy
As a core receptor regulating calcium homeostasis and bone metabolism, PTH1R dysfunction directly links to multiple bone and renal diseases. Drugs targeting PTH1R have advanced from early Teriparatide to new agents like Abaloparatide, with remarkable therapeutic effects. Future research directions include developing more convenient delivery systems, exploring pathway-selective agonists, and expanding applications in renal diseases, liver fibrosis, and other fields. Precision therapies targeting PTH1R are poised to play important roles in a broader range of diseases.
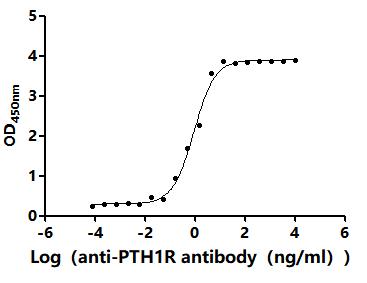
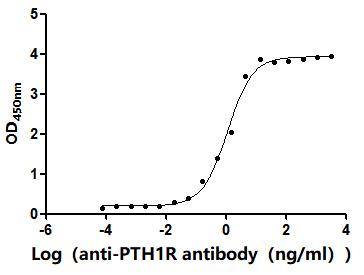
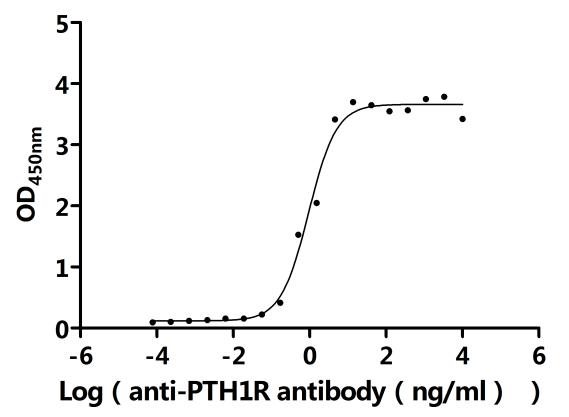
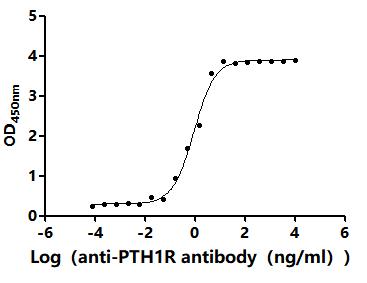
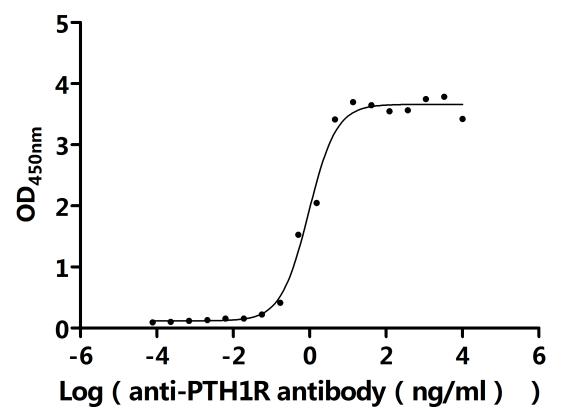
5. CUSABIO's PTH1R-related Products
CUSABIO provides high-quality recombinant proteins and antibodies targeting PTH1R to support mechanistic and translational research:
References
[1] Yongsheng Zhao, Shang Su, Xiaohong Li. Parathyroid Hormone-Related Protein/Parathyroid Hormone Receptor 1 Signaling in Cancer and Metastasis. Cancers. 2023;15 (7):1982-1982.
[2] Eric C. Buxton,Wei Yao,Nancy E Lane. Changes in Serum Receptor Activator of Nuclear Factor-κB Ligand, Osteoprotegerin, and Interleukin-6 Levels in Patients with Glucocorticoid-Induced Osteoporosis Treated with Human Parathyroid Hormone (1–34). The Journal of Clinical Endocrinology & Metabolism. 2004;89 (7):3332-3336.
[3] Stergios A. Polyzos,Polyzois Makras,Zoe Efstathiadou, et al. Investigational parathyroid hormone receptor analogs for the treatment of osteoporosis. Expert Opinion on Investigational Drugs. 2014;24 (2):145-157.
[4] Yi Fan,Jun‐ichi Hanai,Phuong Le, et al. Parathyroid Hormone Directs Bone Marrow Mesenchymal Cell Fate. Cell Metabolism. 2017;25 (3):661-672.
[5] Chi Zhang, Kui Xu, Jianle Wang et al. Parathyroid hormone 1 receptor as a potential therapeutic target for intervertebral disc degeneration, 17 March 2023, PREPRINT (Version 1) available at Research Square.
[6] Alberto Galindo Izquierdo,Pilar López-Luna,Arantxa Ortega, et al. The parathyroid hormone-related protein system and diabetic nephropathy outcome in streptozotocin-induced diabetes. Kidney International. 2006;69 (12):2171-2178.
[7] Yoshikazu Nishimura,Toru Esaki,Yoshiaki Isshiki, et al. Lead Optimization and Avoidance of Reactive Metabolite Leading to PCO371, a Potent, Selective, and Orally Available Human Parathyroid Hormone Receptor 1 (hPTHR1) Agonist. Journal of Medicinal Chemistry. 2020;63 (10):5089-5099.
[8] Srilatha Swami,Hui Zhu,Aria Nisco, et al. Parathyroid hormone 1 receptor signaling mediates breast cancer metastasis to bone in mice. JCI insight. 2023;8 (5):0-0.
CUSABIO team. PTH1R: Key Therapeutic Target in Bone & Metabolic Health | CUSABIO. https://www.cusabio.com/c-21237.html






Comments
Leave a Comment