CRTAM is a recently discovered member of the immunoglobulin superfamily (Ig-SF), a group of proteins crucial for immune system formation, response, and cell interactions. It functions as a cell-surface transmembrane protein, yet research on this target remains limited. Studies have linked CRTAM (CD355) to various conditions like inflammation, asthma, malignant mesothelioma, and certain cancers. It promotes pro-inflammatory cytokine production and might influence the immunopathology of autoimmune diseases. Moreover, CRTAM plays a vital role in Natural Killer (NK) cells' killing of tumor cells, suggesting a potential strategy for targeting tumors. Today, let's delve deeper into this intriguing new member of the Ig-SF family, CRTAM!
1. What is CTRAM?
1.1 CTRAM Structure
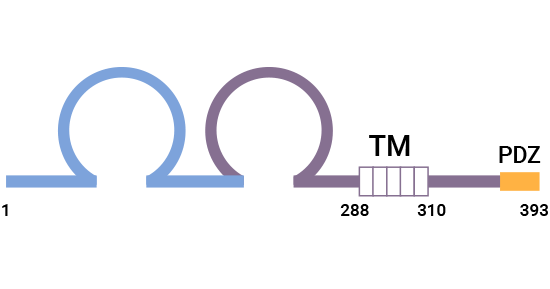
CRTAM (Class I-restricted T cell-associated molecule, also recognized as CD355) is predominantly expressed in activated CD8+ T cells and NKT cells, both restricted by the Class I major histocompatibility complex (MHC), hence its nomenclature. Comprising 393 amino acids, human CRTAM features two immunoglobulin structural domains within its extracellular region-specifically, an IgV followed by an IgC domain. Additionally, its intracellular segment contains PDZ (ESIV)-binding motifs, enabling attachment to the PDZ region of scaffolding proteins. This unique structure aligns CRTAM with the immunoglobulin superfamily (Ig-SF) and also exhibits around 20% sequence similarity to Necl proteins, thereby, also classifying it as a member of the Nectin-like family [1-3].
1.2 CTRAM Expression and Function
CRTAM, a newly identified T cell activation marker, is found on activated iNKT, NK, CD8, and CD4 T cells in lab settings. In mice, CRTAM mRNA is detected in the spleen, brain, and testis; in humans, it's present in multiple tissues including the spleen, thymus, intestines, lymph nodes, lungs, testes, ovaries, and colon. IFN-γ produced by CRTAM+CD4 T cells contributes to inflammation in a mouse model of DSS-induced colitis. In asthma patients, CRTAM is seen in the cell membranes of CD4 and CD8 T cells, neutrophils, and basophils. Additionally, in type 1 diabetes patients, CRTAM expression in iNKT cells correlates significantly with IFN-γ production after antigen-specific stimulation. These findings suggest CRTAM-expressing cells may play roles in various disease-related immune processes [4-6].
Figure 1. CRTAM Structure [1]
2. What is the Ligand for CRTAM?
Nectin-like molecule 2 (Necl-2) has recently emerged as a binding partner for CRTAM. Necl-2, also known by various names including TSLC1, CADM1, SynCAM1, IgSF4, and RAI75, belongs to the Nectin family of proteins (Nectin1-4, Necl1-5). Nectin and Necl molecules facilitate cellular adhesion by forming homo- or heterodimers, influencing adhesion mechanisms. Moreover, they interact with immune molecules such as DNAM-1 (CD226), TIGIT, CD96, CRTAM, and others. Studies employing cell adhesion assays and functional assessments related to NK cells and CD8 cells have identified Necl-2 as the ligand for CRTAM [5, 7-8].
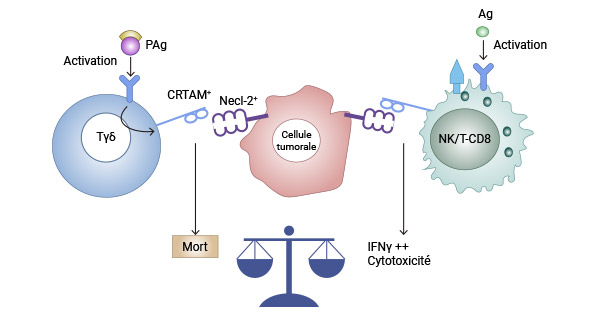
The interaction between CRTAM and its ligand, nectin-like 2 (Necl2), has been observed to significantly impact the production of IL-17, IL-22, and IFN-γ in mouse CD4 T cells. This interaction also plays a pivotal role in optimizing the cytotoxicity of CD8 T cells and NK cells. Studies indicate that the binding of CRTAM to Necl-2 elicits diverse effects depending on the type of lymphocyte involved. In the case of NK or CD8(+) T cells, this interaction promotes cytotoxicity and the secretion of IFNγ, thereby enhancing immune surveillance capabilities. However, the interaction between CRTAM and Necl-2 (TSLC1) triggers cell death specifically in activated Vγ9Vδ2 γδ T cells, potentially facilitating tumor immune escape. (Click to view previous article reports on CRTAM's ligand: DM1/Necl-2/CADM1/TSLC1) [5, 7-9].
Figure 2. The binding of CRTAM to Necl-2 triggers different effects [9]
3. CRTAM-related Regulatory Mechanisms
Recent studies show CRTAM as a versatile protein in immune functions. It collaborates with Necl-2 and scrib, impacting T cell polarity, gamma interferon, and IL-22 secretion [10-12]. Zeb1 has been found to regulate CRTAM expression levels, while other study implied that AP-1 influences CRTAM gene expression in human T CD8 lymphocytes [13-14]. Despite incomplete understanding, Necl-2 and CRTAM interactions play crucial roles in immune processes.
Research findings indicate that CRTAM significantly boosts the cytotoxicity of NK cells against tumor cells. Notably, activated NK cells demonstrated a more pronounced killing effect on tumor cells transfected with Necl-2. In vivo experiments conducted in nude mice using Necl-2-transfected tumor cells resulted in rapid tumor rejection, and the NK cells isolated from these mice notably impeded this rejection when reintroduced [9-11]. In vitro experiments revealed heightened CRTAM expression in CD8+ T cells upon activation by Necl-2-expressing tumor cells, leading to the secretion of gamma interferon post-stimulation. Interfering with the CRTAM-Necl-2 interaction notably suppressed the secretion of gamma interferon. Moreover, Necl-2-presenting antigen-presenting cells stimulated CD8+ T cells to secrete IL-22, contributing to skin cell immunomodulation. The interaction between CRTAM and Necl-2 also plays a crucial role in regulating the retention of CD8+ T cells in lymph nodes. Studies using CRTAM knockout mice revealed reduced numbers of CD8+ T cells, resulting in compromised immunity against viruses [9-11].
4. CRTAM and Disease Research
4.1 CRTAM and Gut Microbiota Research
CRTAM is present in intestinal T cells, regulating their positioning and impacting Th17 cell activity. In mice lacking CRTAM, changes in the intestinal microbiota were observed, with increased Firmicutes and decreased Tenericutes. Comparing wild-type and CRTAM-deficient mice after infection or saline gavage, significant alterations were noted in body weight, fecal composition, and Th17 cell populations. CRTAM supports Th17 cell proliferation and differentiation, thereby improving resistance against intestinal parasites [15].
In addition, CRTAM plays a crucial role in balancing intestinal flora, preventing dysbiosis and inflammation post-infection. In contrast, CRTAM-deficient mice suffered severe weight loss, fecal changes, intestinal damage, dysbiosis, and decreased Th17 cell levels and function after infection. This highlights CRTAM's importance in promoting Th17 cell maturation, protecting against dysbiosis and inflammation due to intestinal parasites in mice. It suggests CRTAM as a potential target for treating such infections [15].
4.2 CRTAM and Asthma Research
The study found increased levels of CD4+CRTAM+ and CD8+CRTAM+ T cells, and CD177+CRTAM+ neutrophils in allergic asthma patients. Eosinophils expressing IL5Rα +CRTAM didn't notably differ. This suggests a potential link between CRTAM in T cells, eosinophils, and neutrophils with bronchial inflammation in allergic asthma [23]. Another study noted a significant relationship between vitamin D levels and genetic variants in the CRTAM gene affecting asthma risk. Examining childhood asthma cohorts revealed three common CRTAM-related variants, particularly rs2272094, associated with increased asthma exacerbations in low vitamin D levels. Further tests showed how vitamin D and rs2272094 influence CRTAM expression. These findings, confirmed in separate populations, underscore the role of vitamin D and CRTAM in CD8+ and natural killer T cells during asthma attacks [16].
4.3 CRTAM and Diabetes Research
CRTAM expression was detected on activated iNKT cells, CD8+ T cells, and a limited subset of CD4+ T cells, showing a correlation with the pro-inflammatory profile of mouse CD4+ T cells. When iNKT cells were stimulated with α-galactoserebroside, CRTAM expression was observed within 18 hours. This indicates that the binding signal of iTCR to α-galactoside alone is enough to trigger CRTAM expression, unaffected by co-stimulatory molecules like CD40, CD80, and CD86. Additionally, researchers noted a significant link between CRTAM expression and the ability of iNKT cells to produce interferon-γ (IFN-γ) in both healthy individuals and type I diabetics. This suggests a potential crucial role of CRTAM in IFN-γ production by human iNKT cells and proposes it as a marker for identifying these inflammatory cells [17].
4.4 Research on CRTAM and Cancer and Other Diseases
In a lung cancer mouse model, researchers discovered that smallpox pollen regulated TSLC1 and CRTAM expression in tumor cells. Blocking TSLC1 expression with small interfering RNA (siRNA) nullified the effects of smallpox pollen on effector T cell proliferation and cytokine secretion, suggesting that smallpox pollen enhances the anti-tumor immune response by promoting TSLC1 and CRTAM interaction [18]. Additionally, CRTAM plays a crucial role in immunomodulating triple-negative breast cancer (TNBC) by boosting immune-inflammatory response and CD8+ T cell infiltration. CRTAM overexpression triggers STAT1 phosphorylation and increases interferon-stimulated genes [19]. Furthermore, CRTAM has been associated with acute lymphoblastic leukemia [20], autoimmune alopecia [21], and tuberculosis [22]. These studies collectively highlight CRTAM's involvement in regulating immune cell behavior and responses, signifying its potential as a novel target for studying various diseases immunologically.
5. Prospects of CRTAM for Clinical Research
HBM-1054, the only current clinical drug targeting CRTAM, is undergoing preclinical formulation development and process optimization and is expected to submit a clinical trial application in 2024. Developed by He-Platinum Pharmaceuticals (Shanghai) Co., Ltd, the drug's clinical indications have not yet been announced, but may involve tumor types with high CTRAM expression, such as lung, breast and gastric cancers. Current clinical drug development and evaluation for CRTAM is still relatively underdeveloped. Therefore, more clinical data are needed to confirm the safety and efficacy of HBM-1054. With in-depth research on CRTAM and its role in tumor immunotherapy, more innovation and collaboration are necessary, which will help expand the understanding of CRTAM and bring more novel research strategies to CRTAM associated diseases.
In Conclusion:
CRTAM, a member of the immunoglobulin superfamily, influences diverse immune processes. Its presence on activated T cells, including iNKT, CD4+, and CD8+ cells, signifies its role in inflammation and disease, impacting conditions like asthma, autoimmune diseases, and certain cancers. Research has unveiled its significance in promoting pro-inflammatory responses, aiding in the fight against tumors by enhancing NK cell-mediated tumor cell killing. Ongoing clinical developments, notably HBM-1054, underscore the potential of targeting CRTAM for novel therapeutic strategies in immunology and cancer research.
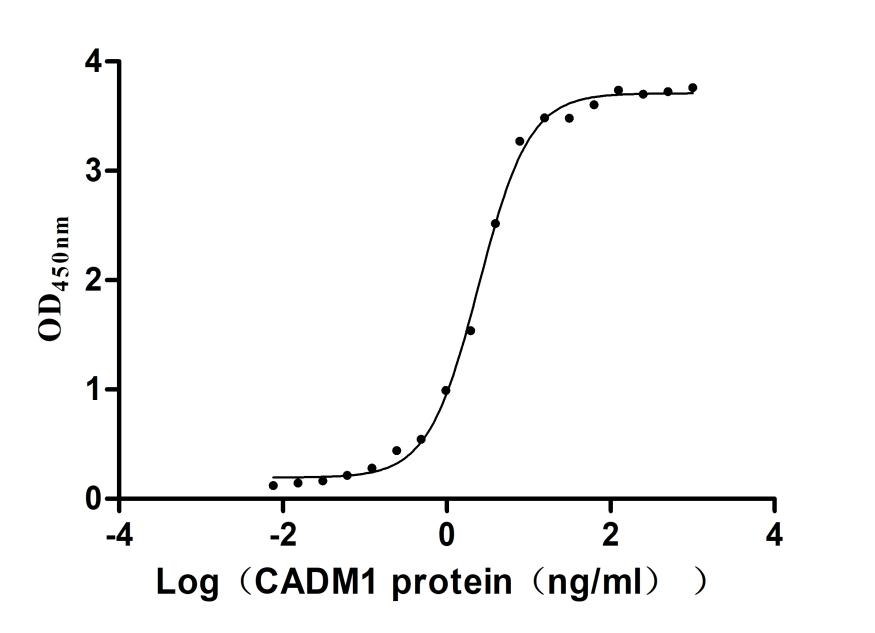
6. CUSABIO CTRAM Recombinant Proteins & Antibodies for Research Use
To fully support researchers and pharmaceutical companies in their research on CTRAM in diseases such as intestinal, asthma, diabetes, or cancers, etc. CUSABIO presents CTRAM active proteins & antibodies to support your research on the mechanism of CTRAM or its potential clinical value.
References
[1] Zhang Shuijun. Study on the molecular mechanism of interaction between NK cell receptors and Nectin/Necl family ligands [D]. China University of Science and Technology, 2014.
[2] Zhang, Shuijun, et al. "Competition of cell adhesion and immune recognition: Insights into the interaction between CRTAM and nectin-like 2." Structure 21.8 (2013): 1430-1439.
[3] Yeh, Jung-Hua, Sachdev S. Sidhu, and Andrew C. Chan. "Regulation of a late phase of T cell polarity and effector functions by Crtam." Cell 132.5 (2008): 846-859.
[4] Garay, Erika, et al. "CRTAM: A molecule involved in epithelial cell adhesion." Journal of cellular biochemistry 111.1 (2010): 111-122.
[5] Arase, Noriko, et al. "Heterotypic interaction of CRTAM with Necl2 induces cell adhesion on activated NK cells and CD8+ T cells." International immunology 17.9 (2005): 1227-1237.
[6] Galibert, Laurent, et al. "Nectin-like protein 2 defines a subset of T-cell zone dendritic cells and is a ligand for class-I-restricted T-cell-associated molecule*♦." Journal of Biological Chemistry 280.23 (2005): 21955-21964.
[7] Boles, Kent S., et al. "The tumor suppressor TSLC1/NECL-2 triggers NK-cell and CD8+ T-cell responses through the cell-surface receptor CRTAM." Blood 106.3 (2005): 779-786.
[8] Dessarthe, Benoît, et al. "CRTAM receptor engagement by Necl-2 on tumor cells triggers cell death of activated Vγ9Vδ2 T cells." The Journal of Immunology 190.9 (2013): 4868-4876.
[9] Nectins and nectin-like receptors DNAM-1 and CRTAM: New ways for tumor escape
[10] Leavy, Olive. "Polarity and CRTAM: a matter of timing." Nature Reviews Immunology 8.4 (2008): 246-246.
[11] Takeuchi, Arata, et al. "CRTAM determines the CD4+ cytotoxic T lymphocyte lineage." Journal of Experimental Medicine 213.1 (2016): 123-138.
[12] YEH, JUNG‐HUA, Sachdev S. Sidhu, and Andrew C. Chan. "Crtam regulates a late phase of T cell polarity and IFNγ/IL22 cytokine production." (2008): 384-384.
[13] Rojas-Marquez, C., et al. "CRTAM is negatively regulated by ZEB1 in T cells." Molecular Immunology 66.2 (2015): 290-298.
[14] Valle-Rios, Ricardo, et al. "Characterization of CRTAM gene promoter: AP-1 transcription factor control its expression in human T CD8 lymphocytes." Molecular immunology 46.16 (2009): 3379-3387.
[15] Perez-Lopez, Araceli, et al. "CRTAM shapes the gut microbiota and enhances the severity of infection." The Journal of Immunology 203.2 (2019): 532-543.
[16] Du, Rose, et al. "Genome-wide association study reveals class I MHC–restricted T cell–associated molecule gene (CRTAM) variants interact with vitamin D levels to affect asthma exacerbations." Journal of allergy and clinical immunology 129.2 (2012): 368-373.
[17] Beristain-Covarrubias, Nonantzin, et al. "Class I-restricted T cell-associated molecule is a marker for IFN-γ-producing iNKT cells in healthy subjects and patients with type 1 diabetes." Journal of Interferon & Cytokine Research 37.1 (2017): 39-49.
[18] Cai, Yuchan, et al. "Trichosanthin enhances anti-tumor immune response in a murine Lewis lung cancer model by boosting the interaction between TSLC1 and CRTAM." Cellular & molecular immunology 8.4 (2011): 359-367.
[19] Zheng, Shuyue, et al. "CRTAM promotes antitumor immune response in triple negative breast cancer by enhancing CD8+ T cell infiltration." (2023).
[20] Ramírez-Ramírez, Dalia, et al. "CRTAM+ NK cells endowed with suppressor properties arise in leukemic bone marrow." Journal of Leukocyte Biology 105.5 (2019): 999-1013.
[21] Giangreco, Adam, et al. "Epidermal Cadm1 expression promotes autoimmune alopecia via enhanced T cell adhesion and cytotoxicity." The Journal of Immunology 188.3 (2012): 1514-1522.
[22] Lai, Rachel PJ, et al. "Transcriptomic characterization of tuberculous sputum reveals a host Warburg effect and microbial cholesterol catabolism." Mbio 12.6 (2021): e01766-21.
[23] Ramirez-Velazquez, Carlos, et al. "Peripheral blood T cells and neutrophils from asthma patients express class-I MHC-restricted T cell-associated molecule." Allergy, Asthma & Clinical Immunology 10 (2014): 1-6.
CUSABIO team. CRTAM: Class I Restricted T cell-associated Molecule, an Intriguing New Member of the Ig-SF Family!. https://www.cusabio.com/c-21153.html



Comments
Leave a Comment