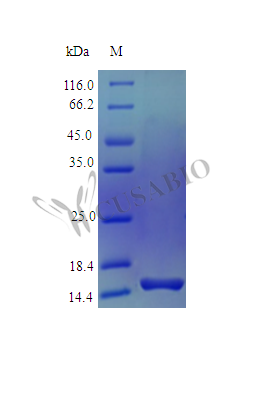
Recombinant Human Pleiotrophin protein (PTN) is produced in an E. coli expression system and represents the full-length mature protein from amino acids 33 to 168. This tag-free protein achieves a high purity level of over 96% as verified by SDS-PAGE. It also maintains a low endotoxin level of less than 1.0 EU/µg according to the LAL method. Its biological activity appears to be confirmed through its ability to enhance neurite outgrowth in rat embryonic cortical neurons under standard testing conditions.
Pleiotrophin is a heparin-binding growth factor that may play a significant role in cellular growth and differentiation. It seems particularly important in neurobiology, where it's known to promote neurite outgrowth and support neuronal development. PTN is involved in several key signaling pathways and has become a subject of interest in neuroscience research due to its potential implications in neural regeneration and repair processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Neurite Outgrowth Assays for Neurodevelopment Research
This recombinant PTN is confirmed to be biologically active in promoting neurite outgrowth in rat embryonic cortical neurons at coating concentrations of 5-10 μg/ml. However, the relatively high working concentration suggests moderate potency compared to some neurotrophic factors. Researchers should validate optimal concentrations for their specific neuronal cultures and consider that the required pre-coating method may yield different results than soluble application. The demonstrated cross-species activity supports use in rodent models, but human neuronal responses should be verified.
2. Protein-Protein Interaction Studies
The tag-free, biologically active PTN is suitable for interaction studies, but the high effective concentration may increase the risk of non-specific binding in sensitive assays. Researchers should include rigorous controls and use appropriate detection methods. The confirmed neurite outgrowth activity indicates proper folding for receptor interactions (e.g., with RPTPβ/ζ), but binding affinity should be quantitatively characterized.
3. Cell Signaling Pathway Analysis
The protein can be used for signaling studies, but the high working concentration and pre-coating requirement may affect signaling kinetics compared to soluble factor addition. Researchers should validate that the signaling pathways (e.g., AKT, ERK) activated by this recombinant PTN match those induced by native PTN, particularly in terms of temporal dynamics and amplitude.
4. Antibody Development and Validation
This high-purity, full-length mature PTN (33-168aa) serves as an excellent antigen for antibody development. The confirmed biological activity ensures antibodies will recognize functional conformational epitopes. However, researchers should validate that antibodies generated against this E. coli-expressed protein also recognize native, mammalian-produced PTN, as the lack of mammalian post-translational modifications might affect some epitopes.
5. Biochemical Characterization and Structure-Function Studies
The protein is suitable for biochemical studies, but researchers should note that the high effective concentration in functional assays may reflect structural properties affecting potency. Biophysical characterization should include assessment of oligomerization state and stability, as PTN's activity can be influenced by its heparin-binding properties and tendency to form multimers.
Final Recommendation & Action Plan
This recombinant human PTN is a validated tool for neurite outgrowth studies, but its relatively high effective concentration (5-10 μg/ml for coating) requires careful experimental design. First, optimize concentrations for your specific application - while coating at 5-10 μg/ml demonstrated activity in rat cortical neurons, soluble application or different cell types may require different concentrations. For interaction studies, the tag-free design is advantageous, but it includes appropriate controls for the high working concentrations. When developing antibodies, this full-length protein is ideal for generating comprehensive antibodies, but validated against native PTN from relevant tissues. For signaling studies, confirm that the pre-coating method activates pathways similarly to soluble PTN in your system. The E. coli expression produces a non-glycosylated protein, which is acceptable as PTN is not heavily glycosylated, but critical findings should be verified with mammalian-expressed PTN when possible. Always include proper controls for the coating procedure itself, as the surface adsorption may affect protein orientation and availability.