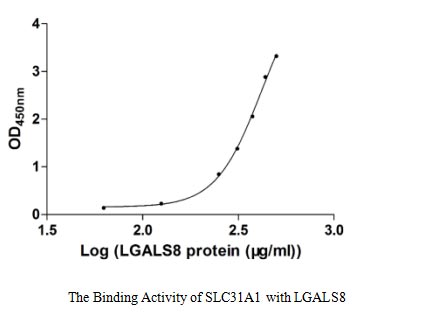
Recombinant Human Galectin-8 (LGALS8) is a full-length protein expressed in E. coli with an N-terminal GST tag. The protein achieves a purity level above 90% as assessed by SDS-PAGE, which appears to provide reliable quality for research applications. Its biological activity has been confirmed through binding capacity in a functional ELISA, where it binds to immobilized SLC31A1 with an EC50 range of 373.90-524.30 μg/ml. This suggests consistent performance in experimental settings.
Galectin-8 belongs to the galectin family, known for binding beta-galactoside sugars. It likely plays important roles in various biological processes, including cell adhesion, migration, and immune response modulation. Galectin-8 appears to be involved in intracellular signaling pathways. Numerous studies have focused on this protein due to its significance in cellular interactions and potential implications in various physiological and pathological processes.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Protein-Protein Interaction Studies with SLC31A1
This recombinant LGALS8 is confirmed to bind SLC31A1 but with very low affinity (EC₅₀ 373.90-524.30 μg/ml), which may not reflect physiological binding kinetics. The high EC₅₀ suggests potential issues with protein folding, activity, or steric hindrance from the N-terminal GST tag. Researchers should validate binding parameters with tag-free LGALS8 or mammalian-expressed protein to ensure biological relevance. The GST tag may facilitate immobilization but could distort interaction studies.
2. GST Pull-Down Assays for Binding Partner Identification
The GST tag enables pull-down assays, but the very high EC₅₀ indicates poor binding functionality, which may lead to false negatives or weak interactions. While the full-length sequence (1-317aa) contains all domains, the E. coli expression lacks glycosylation, and the GST tag may cause non-specific binding. Identified interactors should be validated with native LGALS8 from mammalian cells to confirm physiological relevance.
3. Antibody Development and Validation
This full-length LGALS8 serves as an antigen for antibody development, but the GST tag may induce tag-specific antibodies, reducing antibodies against LGALS8-specific epitopes. The E. coli expression produces a non-glycosylated protein, which may not mimic native glycosylated LGALS8. Antibodies should be validated against mammalian-derived LGALS8 to ensure recognition of physiological forms.
4. Functional ELISA Development and Optimization
The protein can be used for ELISA development, but the very high EC₅₀ (μg/ml range) indicates low sensitivity, making assays impractical for most applications. The GST tag may affect antigen presentation. Researchers should optimize assays with lower EC₅₀ standards or consider tag-free LGALS8 for better performance. The wide EC₅₀ range (373.90-524.30 μg/ml) suggests variability that may affect reproducibility.
5. Biochemical Characterization and Structure-Function Studies
The protein is suitable for basic biochemical studies, but the GST tag may alter protein stability and structure, complicating interpretation. Techniques like circular dichroism may be affected by the tag's presence. The high EC₅₀ suggests possible misfolding or inactivity, so structural conclusions should be validated with tag-free, active LGALS8. E. coli expression lacks glycosylation, which is important for galectin function.
Final Recommendation & Action Plan
This recombinant human LGALS8 with an N-terminal GST tag has confirmed but very weak binding activity (EC₅₀ ~400-500 μg/ml), indicating potential issues with folding or tag interference. It can be used for preliminary studies but requires rigorous validation. For interaction studies, prioritize tag removal or use mammalian-expressed LGALS8 for physiological relevance. For pull-down assays, the GST tag is useful, but include stringent controls (e.g., GST-alone) and validate hits with native protein. For antibody development, this protein can immunize animals but screen clones against glycosylated LGALS8 to avoid tag-specific or non-physiological antibodies. Avoid functional assays requiring high sensitivity due to the low affinity; instead, use it for qualitative binding checks. For biochemical studies, the tag may need cleavage for accurate structural insights. Always confirm key findings with properly modified, full-length LGALS8 expressed in mammalian systems to account for glycosylation and folding. The purity (>90%) is acceptable but may require further purification for sensitive applications. Given the high EC₅₀, this protein is best suited for applications where activity is not critical, such as initial antibody generation or training assays, but not for detailed kinetic or functional studies without further optimization.