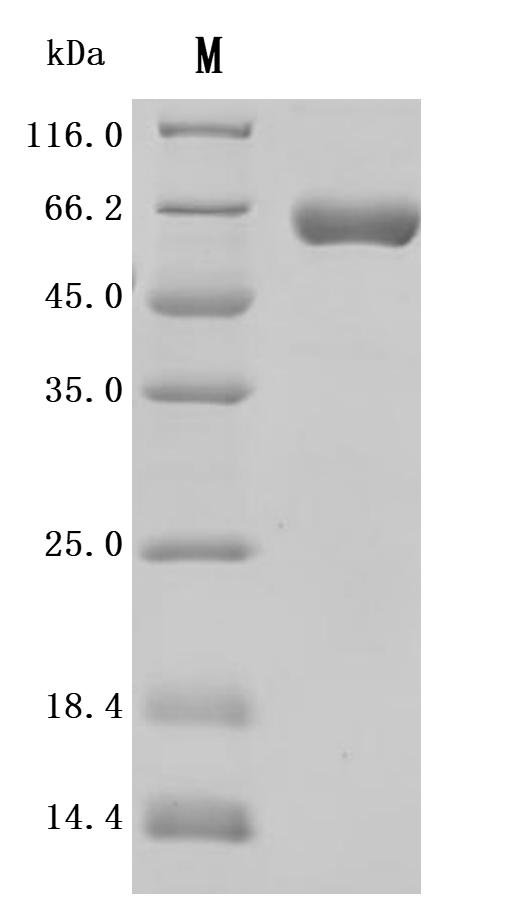
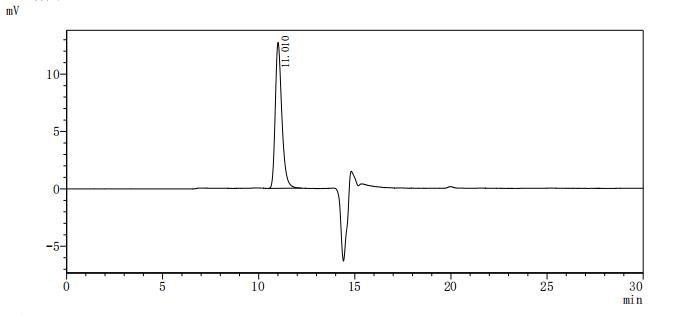
The recombinant Macaca fascicularis ALPP protein is an active enzyme produced in a mammalian expression system to ensure proper folding and native-like post-translational modifications. Spanning amino acids 21 to 504 of the ALPP sequence, it includes a C-terminal 10xHis tag for convenient purification and detection. This lyophilized protein is of high purity, exceeding 95% as verified by both SDS-PAGE and SEC-HPLC. Enzymatic function is confirmed by its catalytic activity. It exhibits a specific activity greater than 7000 U/mg, where one unit is defined as the amount of enzyme needed to hydrolyze 1 nmol of p-nitrophenyl phosphate (pNPP) per minute at 37°C, pH 10.0. These characteristics make it well-suited for biochemical assays, enzymatic studies, and functional research involving alkaline phosphatase activity.
ALPP is an important protein studied in various species, including Macaca fascicularis, commonly known as the cynomolgus monkey or long-tailed macaque. This protein plays vital roles in several physiological processes, such as mineralization, and is particularly critical in the context of pregnancy and placental function.
Evidence indicates that ALPP exhibits distinct expression patterns typical of trophoblast cells, which are important during embryonic development [1]. Immunological studies have shown that ALPP can be detected within specific tissues in cynomolgus monkeys, analogous to findings in humans and other primates [2]. The presence of ALPP can serve as a biomarker in various medical contexts, particularly as a diagnostic tool related to placental health and function [3]. In addition, comparisons of serum ALPP levels have demonstrated varying concentrations between sexes in Macaca fascicularis, with evidence suggesting notably higher activity levels in males [4].
In research focusing on the cynomolgus monkey, it has been observed that variations in ALPP activity can correlate with different physiological states, including stress responses and metabolic changes. For instance, certain conditions can lead to differential expression of genes associated with ALPP in various tissues, highlighting its potential relevance in clinical biomarkers and translational medicine [5]. Furthermore, ALPP's functionality extends beyond enzymatic activity; it also regulates calcium metabolism and can influence the onset of calcific processes in tissues [2]. This enzymatic role underscores the importance of ALPP in exploring skeletal physiology and pathology, particularly in aging and metabolic disorders.
Moreover, the role of ALPP within the broader scope of primate biology is emphasized by studies demonstrating its utility in understanding developmental processes at the cellular and molecular levels. Evidence suggests that cells expressing ALPP share traits with embryonic stem cells and contribute to potential therapeutic avenues in regenerative medicine [6][7]. Thus, the characterization of ALPP in cynomolgus monkeys provides significant insights that underscore its relevance not only within the species but also in comparative studies across primate and human contexts.
References:
[1] R. Kantor, R. Galbraith, D. Emerson, & G. Galbraith. Placental alkaline phosphatase is a major specificity in antisera raised to human trophoblast membranes. American Journal of Reproductive Immunology, vol. 1, no. 6, p. 336-339, 1981. https://doi.org/10.1111/j.1600-0897.1981.tb00068.x
[2] K. Khan, B. Yu, A. H, R. Cecere, & A. Schwertani. Assessing calcification onset in aortic valve interstitial cells. Cardiovascular Pharmacology Open Access, vol. 06, no. 05, 2017. https://doi.org/10.4172/2329-6607.1000222
[3] D. Birkett, J. Done, F. Neale, & S. Posen. Serum alkaline phosphatase in pregnancy: an immunological study. BMJ, vol. 1, no. 5497, p. 1210-1212, 1966. https://doi.org/10.1136/bmj.1.5497.1210
[4] G. Felizardo. Evaluation of the hematological and biochemical profile of leontopithecus chrysomelas (kuhl, 1820) under ex‐situ conditions. Journal of Medical Primatology, vol. 54, no. 2, 2025. https://doi.org/10.1111/jmp.70018
[5] L. Ramsay, M. Quillé, et al. Blood transcriptomic biomarker as a surrogate of ischemic brain gene expression. Annals of Clinical and Translational Neurology, vol. 6, no. 9, p. 1681-1695, 2019. https://doi.org/10.1002/acn3.50861
[6] R. Sánchez‐Pernaute, L. Studer, et al. Long‐term survival of dopamine neurons derived from parthenogenetic primate embryonic stem cells (cyno‐1) after transplantation. Stem Cells, vol. 23, no. 7, p. 914-922, 2005. https://doi.org/10.1634/stemcells.2004-0172
[7] J. Cibelli, K. Grant, K. Chapman, et al. Parthenogenetic stem cells in nonhuman primates. Science, vol. 295, no. 5556, p. 819-819, 2002. https://doi.org/10.1126/science.1065637