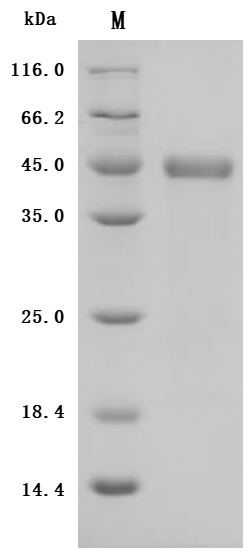
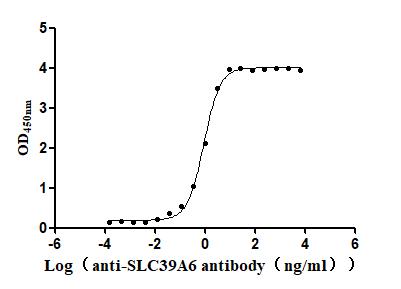
The recombinant Macaca fascicularis SLC39A6 protein is an active, high-purity product expressed using a baculovirus system. It comprises amino acids 21 to 309 of the native SLC39A6 sequence and carries a C-terminal 6xHis tag to facilitate purification and assay compatibility. This recombinant SLC39A6 protein is supplied in lyophilized form and achieves a purity level above 95%, as assessed by SDS-PAGE. Endotoxin contamination is strictly controlled, remaining below 1.0 EU/µg, in line with LAL assay results. Its functional integrity is confirmed through a binding ELISA: when immobilized at 2 μg/mL, it binds specifically to the anti-SLC39A6 recombinant antibody (CSB-RA621669MA1HU), with an EC50 ranging from 0.8290 to 0.9864 ng/mL. These data confirm the SLC39A6 protein's robust activity and suitability for use in binding studies or related assays.
Zinc transporter ZIP6 (SLC39A6) plays a critical role in various physiological processes by regulating zinc homeostasis and contributing to cellular functions in animals. ZIP6 is classified as a member of the LIV-1 subfamily of zinc transporters, which are known to transport zinc across cell membranes, influencing cellular zinc concentrations vital for numerous cellular activities [1][2].
One of the significant functions of ZIP6 is its involvement in epithelial-mesenchymal transition (EMT), a process that is critical in development and cancer progression. Studies highlighted that ZIP6 facilitates the nuclear localization of the zinc-finger transcription factor Snail, which is integral to the regulation of gene expression associated with EMT [1][3]. This mechanism underscores the importance of ZIP6 in promoting cellular migration and invasiveness, linking it to metastasis in various cancers, including triple-negative breast cancer (TNBC) [2].
In the context of immune function, ZIP6 is particularly important for T cell activation. Increased expression of ZIP6 has been shown to enhance cytoplasmic zinc levels, which are crucial for T cell proliferation and survival, especially under stressful conditions such as aging [4][5]. It has been substantiated that zinc influx through ZIP6 is essential for the optimal activation of T lymphocytes, highlighting its pivotal role in maintaining immune health [4].
Additionally, ZIP6 has been associated with metabolic processes, particularly concerning glucose metabolism. High glucose conditions have been noted to stimulate the migration of breast cancer cells, a process mediated by zinc and its transporters, including ZIP6 [6]. This reinforces the understanding that ZIP6 not only contributes to zinc transport but also facilitates cellular responses to environmental cues, such as nutrient availability.
Further, ZIP6's expression is notably higher in cancerous tissues compared to non-cancerous tissues, indicating its potential as a therapeutic target in oncology. Studies have shown that silencing ZIP6 can reduce the malignant properties of cancer cells, suggesting that targeting this transporter may aid in cancer treatment strategies [7].
References:
[1] G. Schmitt‐Ulms, S. Ehsani, J. Watts, D. Westaway, & H. Wille. Evolutionary descent of prion genes from the zip family of metal ion transporters. Plos One, vol. 4, no. 9, p. e7208, 2009. https://doi.org/10.1371/journal.pone.0007208
[2] G. Bianchini, C. Angelis, L. Licata, & L. Gianni. Treatment landscape of triple-negative breast cancer — expanded options, evolving needs. Nature Reviews Clinical Oncology, vol. 19, no. 2, p. 91-113, 2021. https://doi.org/10.1038/s41571-021-00565-2
[3] S. Asayama, S. Nishinohara, & H. Kawakami. Zinc-chelated imidazole groups for dna polyion complex formation. Metallomics, vol. 3, no. 7, p. 680, 2011. https://doi.org/10.1039/c1mt00019e
[4] M. Yu, W. Lee, et al. Regulation of t cell receptor signaling by activation-induced zinc influx. The Journal of Experimental Medicine, vol. 208, no. 4, p. 775-785, 2011. https://doi.org/10.1084/jem.20100031
[5] M. Pae and D. Wu. Nutritional modulation of age-related changes in the immune system and risk of infection. Nutrition Research, vol. 41, p. 14-35, 2017. https://doi.org/10.1016/j.nutres.2017.02.001
[6] T. Takatani‐Nakase, C. Matsui, S. Maeda, S. Kawahara, & K. Takahashi. High glucose level promotes migration behavior of breast cancer cells through zinc and its transporters. Plos One, vol. 9, no. 2, p. e90136, 2014. https://doi.org/10.1371/journal.pone.0090136
[7] Q. Dou. New applications of old metal-binding drugs in the treatment of human cancer. Frontiers in Bioscience-Elite, vol. S4, no. 1, p. 375-391, 2012. https://doi.org/10.2741/s274