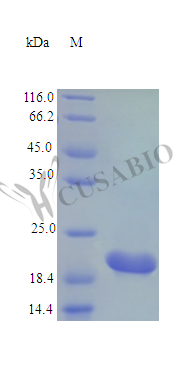
This recombinant Rat Cardiotrophin-1 protein (Ctf1) comes from E. coli production and represents the complete protein sequence, spanning amino acids 1-203. The protein lacks any tags and shows purity greater than 95% when analyzed by SDS-PAGE. It maintains full biological activity, with an ED50 below 0.5 ng/ml in human TF-1 cell proliferation assays—this translates to a specific activity exceeding 2.0 × 10^6 IU/mg. Endotoxin levels stay under 1.0 EU/µg, as measured using the LAL method.
Cardiotrophin-1 appears to function as a cytokine in cell signaling networks, particularly within the interleukin-6 family. It likely plays an important role in how cardiac muscle cells develop and function. The protein seems to activate the JAK/STAT signaling pathway, which may be significant for researchers studying cellular growth and how cells differentiate. This protein could prove valuable for investigations into the molecular mechanisms that drive cardiac physiology and disease.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Cell Proliferation and Viability Assays
This recombinant rat Ctf1 is highly biologically active (ED₅₀ < 0.5 ng/ml) and suitable for proliferation studies in gp130-expressing cells. However, researchers should validate its activity in rat-specific cell systems to confirm that the cross-species activity observed in human TF-1 cells translates to rat physiological contexts. The high specific activity supports reliable dose-response studies, but optimal concentrations may need adjustment for primary rat cardiomyocytes or neural cells, which may have different receptor expression levels.
2. Cytokine Receptor Binding and Signaling Studies
The biologically active Ctf1 is appropriate for studying gp130/LIFR receptor complex interactions and downstream JAK-STAT signaling. The demonstrated activity in human cells suggests conserved receptor binding epitopes, but researchers should validate binding kinetics in rat-specific receptor systems to account for potential species differences. The tag-free design ensures authentic interactions, but signaling amplitude and kinetics should be confirmed in physiologically relevant rat cell types.
3. Antibody Development and Validation
This high-purity, full-length Ctf1 serves as an excellent antigen for antibody development. However, antibodies should be validated against native rat Ctf1 from biological sources to ensure recognition of physiologically relevant forms. The confirmed cross-species bioactivity indicates proper folding of conformational epitopes, supporting the development of function-blocking antibodies. Comprehensive validation should include testing in both human and rat systems to confirm species cross-reactivity
4. Comparative Species Studies and Cross-Reactivity Analysis
The protein enables valid cross-species comparisons, but researchers should note that Ctfl's receptor binding affinity may vary between species due to evolutionary differences in gp130/LIFR complexes. Comparative studies should include parallel assays in the same cell system to eliminate host cell variability. The high potency in human cells suggests strong functional conservation, but quantitative differences in signaling output between rat and human Ctf1 should be empirically determined.
5. Protein-Protein Interaction Studies
The biologically active Ctf1 is suitable for interaction studies with gp130/LIFR receptor components. However, the E. coli expression produces a non-glycosylated protein, which may affect some interactions, as native Ctf1 has N-linked glycosylation sites. Studies focusing on core receptor binding are well-supported, but interactions involving glycosylation-dependent epitopes should be validated with mammalian-expressed Ctf1. The tag-free design minimizes artifacts in binding assays.
Final Recommendation & Action Plan
This recombinant rat Ctf1 is an exceptionally high-quality reagent with demonstrated cross-species bioactivity, making it suitable for all proposed applications with appropriate validation for species-specific effects. For immediate use, employ it at very low concentrations (0.1-1.0 ng/ml) based on the high potency, but establish dose-response curves in your specific cell systems, particularly for rat primary cells where receptor expression may differ from TF-1 cells. When developing antibodies, this full-length protein is ideal for comprehensive epitope coverage, but validate cross-reactivity with native Ctf1 from rat tissues. For signaling studies, the tag-free design ensures authentic receptor interactions, but confirm that signaling kinetics and amplitude match those induced by mammalian-expressed Ctf1 in physiologically relevant systems. The E. coli expression produces a non-glycosylated protein, which is a limitation as native Ctf1 is glycosylated - critical findings should be verified with glycosylated protein when studying glycosylation-dependent functions. For comparative studies, the high specific activity provides an excellent benchmark, but include both rat and human Ctf1 proteins tested in parallel to properly interpret species differences. Always include appropriate controls and consider that different cell types may exhibit varying sensitivity to Ctf1 stimulation based on gp130/LIFR complex expression and composition.