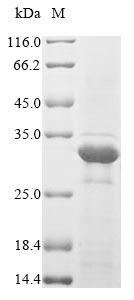
Recombinant Escherichia coli Type 1 fimbrin D-mannose specific adhesin (fimH) is produced in an E. coli expression system and spans the full length of the mature protein from amino acids 22 to 300. The protein includes an N-terminal 6xHis-tag for easier purification and detection. SDS-PAGE analysis confirms a purity level exceeding 85%, which appears suitable for research applications requiring high-quality recombinant proteins.
FimH represents a critical component of type 1 fimbriae in Escherichia coli and plays a significant role in bacterial adherence to host cells. This adhesin specifically binds to D-mannose residues, which allows bacteria to attach to epithelial cells—a key step in pathogenesis. Understanding how fimH interacts with host tissues may be essential for research into bacterial colonization and potential therapeutic interventions.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
The E. coli FimH is a lectin domain protein that requires correct folding and disulfide bond formation for its mannose-binding activity. Since it's expressed in its native host (E. coli), the protein has a high probability of proper folding, as the cellular environment contains the necessary chaperones and oxidative conditions for disulfide bond formation. The N-terminal His tag is small and unlikely to significantly interfere with the lectin domain's function. The high purity further supports good quality. However, without explicit activity validation (e.g., mannose-binding assays), bioactivity cannot be guaranteed, though the probability is high.
1. Antibody Development and Immunoassay Research
This application is well-suited. The recombinant FimH is an excellent immunogen for generating specific antibodies. The His tag facilitates purification and screening. Since it's likely correctly folded, antibodies may recognize conformational epitopes of native FimH. However, validate antibody specificity against wild-type E. coli to confirm recognition of the natural protein in fimbriae.
2. Protein-Protein Interaction Studies
The His tag enables pull-down assays to identify interaction partners (e.g., other fimbrial subunits like FimG/FimF, or host receptors). Given the high likelihood of correct folding, interactions are likely physiological. However, the tag could theoretically cause steric hindrance. Validate critical interactions using an alternative method (e.g., SPR with tag-free protein or genetic approaches).
3. Biochemical Characterization and Binding Assays
This application is highly feasible and is a critical first step for validation. Use this protein in binding assays (e.g., SPR, MST) with D-mannose or glycoproteins to confirm its lectin activity. The high purity is suitable for quantitative studies. If activity is confirmed, it can be reliably used for kinetic studies (e.g., determining Kd for mannose).
4. Vaccine Research and Immunogenicity Studies
This application is promising but requires caution. The recombinant protein can be used as a vaccine antigen to generate antibodies that block adhesion. However, the immune response should be evaluated for its ability to inhibit bacterial adhesion to host cells, which is the functional goal of an anti-adhesin vaccine. Assess if antibodies prevent binding to mannosylated receptors in cell-based assays.
Final Recommendation & Action Plan
This recombinant FimH has a high probability of being correctly folded and bioactive due to its expression in its native host, E. coli. The immediate priority is to experimentally validate its mannose-binding activity using a simple assay like yeast cell agglutination, SPR, or ELISA with mannosylated BSA. Once activity is confirmed, the protein can be confidently used for interaction studies, detailed biochemical characterization, and immunogenicity assessments. For vaccine studies, focus on functional inhibition assays to ensure elicited antibodies are neutralizing.