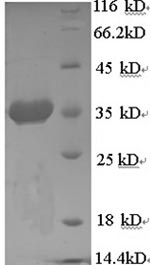
Steps for preparing the recombinant human endoplasmic reticulum chaperone BiP (HSPA5) protein include synthesizing the gene that encodes the partial HSPA5 protein (25-293aa) and co-cloning the gene in a suitable vector with the N-terminal 6xHis-tag gene, followed by transforming the constructed vector into E.coli cells for protein expression. The obtained protein is purified from the cell lysate through affinity chromatography. Its purity is up to 90% as measured by SDS-PAGE.
HSPA5, also known as BiP or GRP78, is a crucial protein belonging to the Hsp70 family. It acts as a master chaperone protein primarily located in the endoplasmic reticulum (ER). HSPA5 plays a vital role in protein folding processes by responding to the accumulation of misfolded or unfolded proteins in the ER [1][2]. It interacts with other chaperone proteins like DNAJC10 to facilitate correct protein folding or degradation of misfolded proteins [1]. Moreover, HSPA5 regulates gene expression and alternative splicing related to inflammatory and immune responses [3].
Research indicates that HSPA5 is a calcium-binding protein that influences calcium transfer between the ER and mitochondria, thereby contributing to maintaining mitochondrial function [3]. HSPA5 has been identified as a key player in various diseases, such as nonalcoholic fatty liver disease and cancer [2][4]. Studies have shown that HSPA5 can bind directly to specific proteins like TDP-43, mitigating toxicity associated with neurodegenerative diseases [5][6]. Additionally, HSPA5 has been linked to apoptosis regulation, making it a potential target for cancer therapies [7].
References:
[1] T. Li, New progresses on cell surface protein hspa5/bip/grp78 in cancers and covid-19, Frontiers in Immunology, vol. 14, 2023. https://doi.org/10.3389/fimmu.2023.1166680
[2] A. Rehati, B. Abuduaini, L. Zhao, D. Chen, & F. He, Identification of heat shock protein family a member 5 (hspa5) targets involved in nonalcoholic fatty liver disease, Genes and Immunity, vol. 24, no. 3, p. 124-129, 2023. https://doi.org/10.1038/s41435-023-00205-y
[3] H. Fan, L. Xue, Y. Liu, D. Zuo, F. Gao, H. Liet al., Hspa5 regulates the expression and alternative splicing of inflammatory and immune response genes.,, 2021. https://doi.org/10.21203/rs.3.rs-397944/v1
[4] L. Booth, J. Roberts, & P. Dent, Hspa5/dna k may be a useful target for human disease therapies, Dna and Cell Biology, vol. 34, no. 3, p. 153-158, 2015. https://doi.org/10.1089/dna.2015.2808
[5] L. François-Moutal, D. Scott, A. Ambrose, C. Zerio, K. Dissanayake, D. Mayet al., Heat shock protein grp78/bip/hspa5 binds directly to tdp-43 and mitigates toxicity associated with neurodegenerative disease pathology,, 2021. https://doi.org/10.21203/rs.3.rs-1090289/v1
[6] L. François‐Moutal, D. Scott, A. Ambrose, C. Zerio, M. Rodriguez-Sanchez, K. Dissanayakeet al., Heat shock protein grp78/bip/hspa5 binds directly to tdp-43 and mitigates toxicity associated with disease pathology, Scientific Reports, vol. 12, no. 1, 2022. https://doi.org/10.1038/s41598-022-12191-8
[7] F. Uckun, S. Qazi, Z. Ozer, A. Garner, J. Pitt, H. Maet al., Inducing apoptosis in chemotherapy‐resistant b‐lineage acute lymphoblastic leukaemia cells by targeting hspa5, a master regulator of the anti‐apoptotic unfolded protein response signalling network, British Journal of Haematology, vol. 153, no. 6, p. 741-752, 2011. https://doi.org/10.1111/j.1365-2141.2011.08671.x
[8] M. Komatsu, P62 bodies: phase separation, nrf2 activation, and selective autophagic degradation, Iubmb Life, vol. 74, no. 12, p. 1200-1208, 2022. https://doi.org/10.1002/iub.2689
[9] D. Lee, D. Kim, S. Kim, S. Jeong, J. Kim, S. Shimet al., Park7 modulates autophagic proteolysis through binding to the n-terminally arginylated form of the molecular chaperone hspa5, Autophagy, vol. 14, no. 11, p. 1870-1885, 2018. https://doi.org/10.1080/15548627.2018.1491212