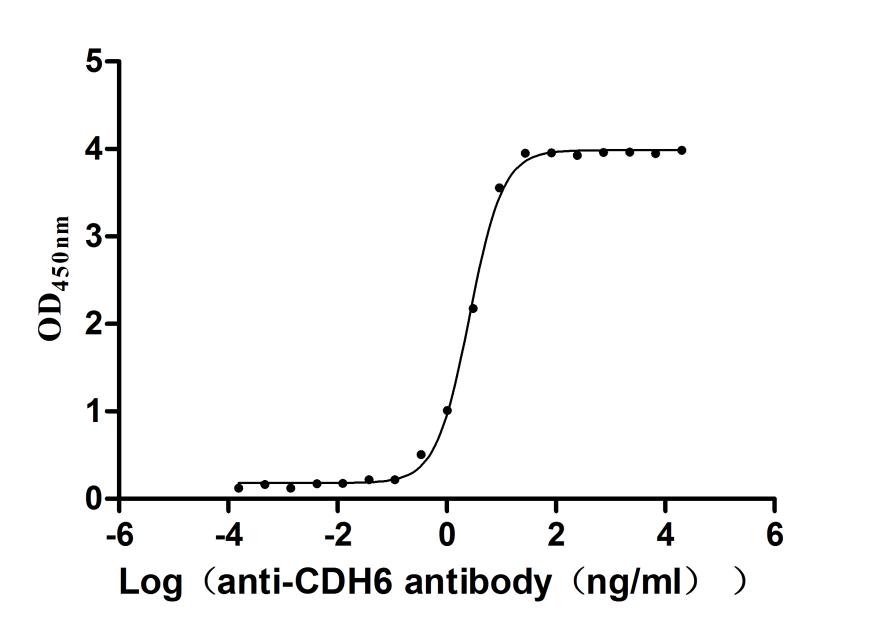
This recombinant human cadherin-6 (CDH6) protein, spanning the extracellular domain (54-615aa), is produced in mammalian cells with a C-terminal 10×His tag. The protein exhibits high purity (>95% by SEC-HPLC) and low endotoxin levels (<1.0 EU/μg, LAL method). It has been validated for functional studies. ELISA demonstrates that it strongly binds to anti-CDH6 recombinant antibody (CSB-RA005055MA1HU), with the EC50 of 2.421–2.802 ng/mL, confirming structural integrity and antigenic specificity. The His tag facilitates purification without disrupting adhesive interfaces. Its validated activity makes it ideal for antibody validation, cell adhesion assays, and studies of CDH6's involvement in tumor progression and tissue morphogenesis.
Human CDH6 is a member of the cadherin superfamily of proteins, which are essential cell-cell adhesion molecules. As a type II cadherin, CDH6 is characterized by its single transmembrane domain and five extracellular repeats, facilitating calcium-dependent interactions crucial for maintaining tissue architecture and integrity [1]. CDH6 plays a significant role in various biological contexts, particularly in the central nervous system and kidneys, where it supports the formation of adherens junctions and is involved in cellular signaling pathways related to development and disease progression [2][3].
In the human endometrium, CDH6 is localized on the lateral surfaces of epithelial cells, where it is crucial for establishing junctions necessary for maintaining the integrity of the endometrial luminal epithelium during the luteal phase of the menstrual cycle. Its expression and localization are pivotal during embryo implantation, as CDH6 may undergo redistribution to facilitate the attachment and invasion of the embryo [2]. Furthermore, in the context of renal physiology, CDH6 is expressed in the proximal renal tubule, indicating its involvement in kidney function and potentially in renal pathology [1][4].
Beyond its structural roles, research indicates that CDH6 is implicated in cancer biology. Its expression has been associated with various malignancies, including ovarian and renal cancers, where it not only serves as an adhesion molecule but also participates in pathways that drive epithelial-to-mesenchymal transition (EMT), a critical process in cancer metastasis [5][6]. Notably, targeting CDH6 with antibody-drug conjugates shows promise in therapeutic applications, particularly for treating tumors expressed in tissues where CDH6 is overrepresented [3][7][8].
Immunohistochemical analyses have demonstrated that CDH6 is expressed in diverse structures within the glomeruli of the kidneys [9][10]. The protein's expression profile reveals its potential utility as a biomarker for renal disease and as a target for innovative immunotherapeutic strategies, particularly given its association with proteinuria and loss of kidney function [10][11]. Recent studies have identified CDH6 as part of a signature gene set associated with podocyte health and the glomerular disease response, highlighting its clinical relevance in nephrology [12].
References:
[1] H. Suzuki, S. Nagase, et al. Raludotatug deruxtecan, a cdh6-targeting antibody–drug conjugate with a dna topoisomerase i inhibitor dxd, is efficacious in human ovarian and kidney cancer models. Molecular Cancer Therapeutics, vol. 23, no. 3, p. 257-271, 2024. https://doi.org/10.1158/1535-7163.mct-23-0287
[2] W. Zhou, L. Santos, & E. Dimitriadis. Characterization of the role for cadherin 6 in the regulation of human endometrial receptivity., Reproductive Biology and Endocrinology, vol. 18, no. 1, 2020. https://doi.org/10.1186/s12958-020-00624-w
[3] T. Li, P. Chen, et al. 1069 atg-106, a novel ‘2+1’ format cdh6-targeted t-cell engager (tce), shows potent t cell dependent cytotoxicity and in vivo anti-tumor efficacy. p. A1189-A1189, 2024. https://doi.org/10.1136/jitc-2024-sitc2024.1069
[4] J. Keeling and G. Falchook. Oncology clinical trials targeting members of the cadherin superfamily: a review. Journal of Immunotherapy and Precision Oncology, vol. 8, no. 1, p. 23-33, 2025. https://doi.org/10.36401/jipo-24-20
[5] R. Bartolomé, J. Robles, et al. Cdh6‐activated αiibβ3 crosstalks with α2β1 to trigger cellular adhesion and invasion in metastatic ovarian and renal cancers. Molecular Oncology, vol. 15, no. 7, p. 1849-1865, 2021. https://doi.org/10.1002/1878-0261.12947
[6] S. Fasih, S. Welch, & A. Lohmann. Antibody–drug conjugates: a start of a new era in gynecological cancers. Current Oncology, vol. 31, no. 11, p. 7088-7106, 2024. https://doi.org/10.3390/curroncol31110522
[7] A. Mahmoud, R. Nabavizadeh, et al. Antibody-based therapeutics for the treatment of renal cell carcinoma: challenges and opportunities. The Oncologist, vol. 28, no. 4, p. 297-308, 2023. https://doi.org/10.1093/oncolo/oyac263
[8] Y. Chen, B. Rini, & K. Beckermann. Emerging targets in clear cell renal cell carcinoma. Cancers, vol. 14, no. 19, p. 4843, 2022. https://doi.org/10.3390/cancers14194843
[9] S. Gharib, J. Pippin, T. Ohse, S. Pickering, R. Krofft, & S. Shankland. Transcriptional landscape of glomerular parietal epithelial cells. Plos One, vol. 9, no. 8, p. e105289, 2014. https://doi.org/10.1371/journal.pone.0105289
[10] J. Harder, R. Menon, et al. Organoid single cell profiling identifies a transcriptional signature of glomerular disease. Jci Insight, vol. 4, no. 1, 2019. https://doi.org/10.1172/jci.insight.122697
[11] A. Nunez-Nescolarde, D. Nikolic‐Paterson, & A. Combes. Human kidney organoids and tubuloids as models of complex kidney disease. American Journal of Pathology, vol. 192, no. 5, p. 738-749, 2022. https://doi.org/10.1016/j.ajpath.2022.01.009
[12] J. Harder, R. Menon, et al., Organoid single-cell profiling identifies a transcriptional signature of glomerular disease. 2018. https://doi.org/10.1101/468850