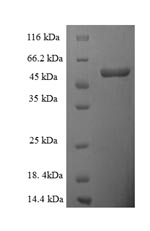
Recombinant Human D-amino-acid oxidase (DAO) gets expressed in E. coli and matches the complete amino acid sequence (1-347aa) found in the human protein. This product comes with an N-terminal 6xHis-SUMO tag, which makes purification and detection more straightforward. The protein reaches greater than 90% purity, as confirmed by SDS-PAGE analysis. This appears to provide high-quality material suitable for research work. Worth noting - this product is meant strictly for research purposes.
D-amino-acid oxidase (DAO) is an enzyme that handles the oxidative deamination of D-amino acids, turning them into their corresponding imino acids. The enzyme likely plays an important role in amino acid breakdown and participates in several metabolic pathways. DAO has caught researchers' attention, particularly those studying its function in brain processes and its possible connections to neurological disorders.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Human DAO is a flavoprotein that requires binding to the cofactor FAD (flavin adenine dinucleotide) for enzymatic activity. While the SUMO tag may promote soluble expression and correct folding in E. coli, the presence of the tag and the need for FAD incorporation mean that correct folding and bioactivity are not guaranteed. E. coli can sometimes produce active flavoproteins, but it often requires optimization (e.g., co-expression of FAD biosynthesis pathways or in vitro FAD reconstitution). Without experimental validation (e.g., enzyme activity assays), it is uncertain whether the protein is correctly folded and bioactive. Therefore, while there is a possibility of activity, it cannot be assumed without verification.
1. Enzyme Kinetics and Substrate Specificity Studies
If verified to be enzymatically active (e.g., through FAD binding and activity assays), this recombinant human DAO can be used for characterizing the enzyme's kinetic parameters and substrate preferences under controlled lab conditions. Scientists can track enzyme activity using different D-amino acid substrates to determine Km, Vmax, and kcat values. The high purity (>90%) helps ensure accurate kinetic measurements by reducing interference from impurities. The N-terminal His-SUMO tag facilitates purification and allows for immobilization in repeated testing, but the tag should be assessed for potential interference with enzyme activity. If misfolded/inactive (unverified), kinetic measurements will yield biologically meaningless results. The large SUMO tag may sterically interfere with substrate access to the active site.
2. Inhibitor Screening and Drug Discovery Research
Provided that the protein is confirmed to be bioactive, the purified DAO can serve as a target for testing potential inhibitors in pharmaceutical research. Small molecule libraries can be screened against the enzyme to identify compounds that modulate DAO activity. The His-tag enables simple protein capture in high-throughput screening setups. However, without activity validation, results may be misleading due to potential misfolding or lack of FAD binding.
3. Antibody Development and Validation
This recombinant human DAO protein can be used as an antigen for generating antibodies, as antibody production often tolerates some misfolding. The high purity is suitable for immunization protocols. It may also function as a positive control in Western blotting, ELISA, and immunoprecipitation, but antibodies raised against misfolded protein might not recognize native DAO, so caution is advised for applications requiring specificity to the native conformation.
4. Protein-Protein Interaction Studies
If the protein is correctly folded, the His-SUMO tagged DAO could be used in pull-down assays to identify binding partners. The dual tagging offers flexibility for capture methods. However, if misfolded, interaction results may not reflect physiological relevance, as binding sites could be altered. Folding should be verified before such studies.
5. Structural and Biophysical Characterization
The high purity and full-length nature make this protein suitable for biophysical analyses (e.g., circular dichroism, dynamic light scattering) to assess folding and stability. However, for detailed structural studies (e.g., X-ray crystallography), correct folding is essential. A misfolded protein may not yield meaningful data. Preliminary checks (e.g., spectroscopy) are recommended to confirm conformation.
Final Recommendation & Action Plan
Before using this recombinant DAO protein for any functional application, it is crucial to experimentally validate its folding and bioactivity. Start by performing a simple enzyme activity assay using a D-amino acid substrate (e.g., D-serine) and monitoring FAD-dependent oxidation, which will confirm if the protein is active. If activity is low, consider in vitro FAD reconstitution or tag removal via SUMO protease. For non-functional uses (e.g., antibody production), proceed with caution and validate antibodies against native DAO. Always include appropriate controls in your experiments to account for potential folding issues.