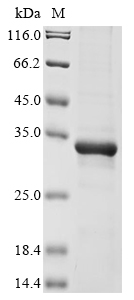
Recombinant Human Meteorin (METRN) is produced in E.coli and includes the full length of the mature protein, corresponding to amino acids 24-293. The protein features an N-terminal 6xHis tag for easier purification and detection. With a purity level exceeding 85% as confirmed by SDS-PAGE, this product appears suitable for research applications requiring high-quality protein samples.
Meteorin is a secreted protein known for its involvement in neural development and repair processes. It may play a role in promoting the differentiation of glial cells and angiogenesis, making it a significant focus in studies related to neurobiology and vascular biology. Researchers often turn to Meteorin to explore pathways critical for nervous system function and potential therapeutic applications.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Meteorin is a human secreted protein that typically requires proper disulfide bond formation and specific tertiary structure for its biological activity in glial cell differentiation and neuroprotection. E. coli expression systems cannot provide the eukaryotic oxidative environment necessary for correct disulfide bond formation and often fail to properly fold complex eukaryotic proteins with multiple cysteine residues. No validation data (e.g., cell-based activity assays, circular dichroism) are provided. Therefore, the protein's folding status and bioactivity cannot be confirmed and are likely inadequate for functional studies.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
This application carries substantial risk without folding validation. If correctly folded, the His-tagged METRN could theoretically be used to identify binding partners, but the bacterial expression system is unlikely to produce a properly folded protein capable of specific interactions. If misfolded (highly probable in E. coli), the protein will exhibit non-specific binding patterns that do not reflect biological interactions. The lack of native post-translational modifications further compromises interaction authenticity.
2. Antibody Development and Validation
This application is suitable regardless of folding status. The recombinant METRN can serve as an effective immunogen for antibody generation, as antibodies primarily recognize linear epitopes. The minimal post-translational modifications in E. coli may actually be advantageous for producing antibodies targeting the core protein sequence. However, antibodies generated against misfolded protein may not optimally recognize conformation-dependent epitopes of native METRN in biological systems.
3. Structural and Biophysical Characterization Studies
This application requires proper folding validation. If correctly folded, the protein could be used for limited structural characterization. However, if misfolded, biophysical data would misrepresent the native protein's properties. The characterization would primarily reflect the misfolded state rather than providing insights into biologically relevant METRN structure.
4. Cell-Based Binding and Uptake Assays
This application is not recommended without folding and functional validation. Cell-based binding studies require a properly folded protein with maintained structural integrity for biologically relevant interactions. If misfolded, the protein will not exhibit correct binding specificity or cellular uptake, making experimental results biologically meaningless. Tag-mediated detection may work technically, but cannot compensate for functional deficiency.
Final Recommendation & Action Plan
This E. coli-expressed His-tagged METRN is unsuitable for functional studies without rigorous validation due to the high probability of misfolding in prokaryotic systems. Before any application, protein folding must be validated through biophysical methods (circular dichroism, size-exclusion chromatography) and functional activity confirmed using cell-based assays. For reliable results, consider alternative eukaryotic expression systems (mammalian or insect cells) that support proper disulfide bond formation and post-translational modifications. Antibody development can proceed with the understanding that resulting antibodies may require additional validation for native protein recognition. Functional studies (applications 1 and 4) should be avoided unless proper folding and bioactivity are conclusively demonstrated.