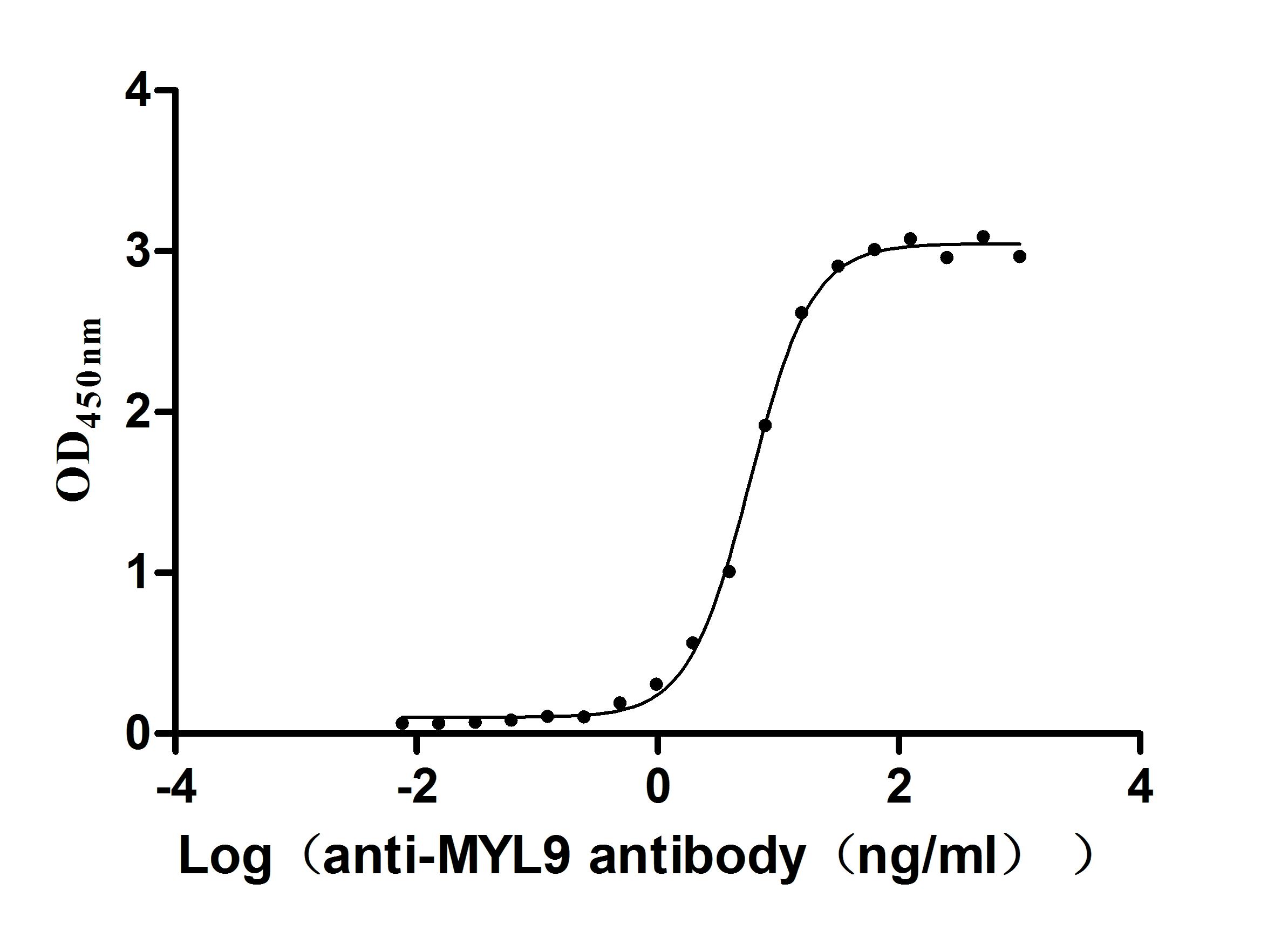
This recombinant human MYL12A protein (amino acid residues 1-171) is produced in E. coli with a C-terminal 6×His tag, achieving high purity (>95% by SDS-PAGE) and low endotoxin content (<1.0 EU/μg, LAL method). Activity assessment demonstrates strong cross-reactivity with anti-MYL9 antibody (CSB-RA015318MA1HU) in ELISA (EC50: 5.325–6.456 ng/mL at 2 μg/mL immobilization), highlighting structural similarities within the myosin light chain family. The lyophilized format guarantees stability and easy reconstitution for biochemical assays. The His tag enables efficient purification without disrupting functional motifs. This recombinant MYL12A protein serves as a vital reagent for investigating actomyosin interactions, mechanobiology, and pathologies linked to myosin regulatory pathway dysregulation, such as cardiovascular disorders.
The human MYL12A protein, also known as MRLC3, is a crucial component of non-muscle myosin II B (NMIIB) and plays a significant role in cellular dynamics, particularly in muscle contraction and cytoskeletal organization. MYL12A is part of the myosin light chain family, contributing to the regulation of actomyosin contraction, which facilitates various cellular functions from muscle contraction to motility and shape changes in non-muscle cells [1][2].
Expression of MYL12A is found in various tissue types, prominently in the heart, where it is essential for proper cardiac function. It is implicated in the formation of cardiac progenitor cells and the overall development of cardiac structures within the ventricles [3]. Studies indicate that MYL12A is upregulated in conditions such as Pompe disease, characterized by hypertrophic cardiomyopathy, suggesting its role in cardiac hypertrophy [3][2]. Moreover, the interaction of MYL12A with other proteins is fundamental in various physiological processes, such as focal adhesion and the regulation of the actin cytoskeleton [2].
MYL12A has been identified as a biomarker in the context of cardiovascular diseases. Genome-wide association studies have recognized MYL12A as a candidate gene for cardiovascular risk, emphasizing its potential involvement in heart disease pathogenesis [1]. Additionally, mutations or dysregulation of MYL12A expression can lead to changes in cellular morphology and dynamics, affecting processes such as migration and proliferation in different cancer types, where it is overexpressed [4][5].
In addition to its myogenic functions, emerging evidence suggests that MYL12A is involved in other cellular processes, such as exosome biogenesis during cardiac differentiation, highlighting its importance beyond traditional muscle biology [3]. It has also been noted for its role in cellular responses to various stimuli, including potential responses to viral infections, where it contributes to cytoskeletal remodeling [6].
References:
[1] Y. Dong, R. Lu, et al. Deficiency in prader-willi syndrome gene necdin leads to attenuated cardiac contractility. Iscience, vol. 27, no. 6, p. 109974, 2024. https://doi.org/10.1016/j.isci.2024.109974
[2] S. Voskamp, M. Hammonds, T. Knapp, A. Pekmezian, D. Hadley, & J. Nelson. meta‐analysis reveals differential gene expression in tetralogy of fallot versus controls. Birth Defects Research, vol. 116, no. 1, 2023. https://doi.org/10.1002/bdr2.2293
[3] P. Ashok and E. Tzanakakis. Proteomic analysis of exosomes during cardiogenic differentiation of human pluripotent stem cells. Cells, vol. 10, no. 10, p. 2622, 2021. https://doi.org/10.3390/cells10102622
[4] L. Korrodi‐Gregório, V. Soto‐Cerrato, R. Vitorino, M. Fardilha, & R. Pérez‐Tomás. From proteomic analysis to potential therapeutic targets: functional profile of two lung cancer cell lines, a549 and sw900, widely studied in pre-clinical research. Plos One, vol. 11, no. 11, p. e0165973, 2016. https://doi.org/10.1371/journal.pone.0165973
[5] Y. Dai, M. Zhang, et al. Salmonella manipulates macrophage migration via stec-mediated myosin light chain activation to penetrate the gut-vascular barrier. The Embo Journal, vol. 43, no. 8, p. 1499-1518, 2024. https://doi.org/10.1038/s44318-024-00076-7
[6] A. Hunziker, I. Glas, M. Pohl, & S. Stertz. Phosphoproteomic profiling of influenza virus entry reveals infection-triggered filopodia induction counteracted by dynamic cortactin phosphorylation. Cell Reports, vol. 38, no. 4, p. 110306, 2022. https://doi.org/10.1016/j.celrep.2022.110306