[1] Cao, Lichuang, et al. "Phosphorylation of myosin regulatory light chain affects actomyosin dissociation and myosin degradation." International Journal of Food Science & Technology 54.6 (2019): 2246-2255.
[2] Gao, Xing, et al. "Dephosphorylation of myosin regulatory light chain modulates actin–myosin interaction adverse to meat tenderness." International Journal of Food Science & Technology 52.6 (2017): 1400-1407.
[3] Cao, Lichuang, et al. "Phosphorylation of myosin regulatory light chain at Ser17 regulates actomyosin dissociation." Food Chemistry 356 (2021): 129655.
[4] Aguilar-Cuenca, Rocío, et al. "Tyrosine phosphorylation of the myosin regulatory light chain controls non-muscle myosin II assembly and function in migrating cells." Current Biology 30.13 (2020): 2446-2458.
[5] Sweeney, H. Lee, and Erika LF Holzbaur. "Motor proteins." Cold Spring Harbor Perspectives in Biology 10.5 (2018): a021931.
[6] Mackay, Charles Edward. SrcFK is a key mediator of oxidant signalling pathways in Pulmonary Vascular Smooth Muscle. Diss. King's College London, 2016.
[7] Haraguchi, Takeshi, et al. "Discovery of ultrafast myosin, its amino acid sequence, and structural features." Proceedings of the National Academy of Sciences 119.8 (2022): e2120962119.
[8] Kumar, C. Chandra, et al. "Characterization and differential expression of human vascular smooth muscle myosin light chain 2 isoform in nonmuscle cells." Biochemistry 28.9 (1989): 4027-4035.
[9] Wang, Shaoxun, et al. "Down‐Regulation of Gamma‐Adducin Disrupts the Actin Cytoskeleton in FHH rats and May Contribute to the Development of Hypertension‐induced Renal Injury." The FASEB Journal 32 (2018): 721-10.
[10] Dabrowska, Magdalena, Marek Skoneczny, and Wojciech Rode. "Functional gene expression profile underlying methotrexate-induced senescence in human colon cancer cells." Tumor Biology 32 (2011): 965-976.
[11] Jiang, Yuhui, et al. "PKM2 phosphorylates MLC2 and regulates cytokinesis of tumour cells." Nature communications 5.1 (2014): 5566.
[12] Iwasaki, Takahiro, et al. "Diphosphorylated MRLC is required for organization of stress fibers in interphase cells and the contractile ring in dividing cells." Cell structure and function 26.6 (2001): 677-683.
[13] Gutjahr, Marc C., Jérémie Rossy, and Verena Niggli. "Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells." Experimental cell research 308.2 (2005): 422-438.
[14] Parker, Robert, et al. "Phosphoproteomic analysis of cell-based resistance to BRAF inhibitor therapy in melanoma." Frontiers in oncology 5 (2015): 95.
[15] Wang, Shibo, et al. "Myosin light chain kinase mediates intestinal barrier dysfunction following simulated microgravity based on proteomic strategy." Journal of proteomics 231 (2021): 104001.
[16] Hayashizaki, Koji, et al. "Myosin light chains 9 and 12 are functional ligands for CD69 that regulate airway inflammation." Science immunology 1.3 (2016): eaaf9154-eaaf9154.
[17] Kimura, Motoko Y., et al. "A new therapeutic target: the CD69-Myl9 system in immune responses." Seminars in immunopathology. Vol. 41. Springer Berlin Heidelberg, 2019.
[18] Yokoyama, Masaya, et al. "Myosin light chain 9/12 regulates the pathogenesis of inflammatory bowel disease." Frontiers in Immunology 11 (2021): 594297.
[19] Sun, Jie, et al. "Distinct roles of smooth muscle and non-muscle myosin light chain-mediated smooth muscle contraction." Frontiers in Physiology 11 (2020): 593966.
[20] Isobe, Kiyoshi, et al. "CRISPR-Cas9/phosphoproteomics identifies multiple noncanonical targets of myosin light chain kinase." American Journal of Physiology-Renal Physiology 318.3 (2020): F600-F616.
[21] Orgaz, Jose L., et al. "Myosin II reactivation and cytoskeletal remodeling as a hallmark and a vulnerability in melanoma therapy resistance." Cancer Cell 37.1 (2020): 85-103.
[22] Jin, Younggeon, and Anthony T. Blikslager. "The regulation of intestinal mucosal barrier by myosin light chain kinase/rho kinases." International Journal of Molecular Sciences 21.10 (2020): 3550.
[23] Kaibuchi, Kozo, Shinya Kuroda, and Mutsuki Amano. "Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells." Annual review of biochemistry 68.1 (1999): 459-486.
[24] Scruggs, Sarah B., and R. John Solaro. "The significance of regulatory light chain phosphorylation in cardiac physiology." Archives of biochemistry and biophysics 510.2 (2011): 129-134.
[25] Ito, Masaaki, et al. "Regulation of myosin light-chain phosphorylation and its roles in cardiovascular physiology and pathophysiology." Hypertension Research 45.1 (2022): 40-52.
[26] Ding, Peiguo, et al. "Cardiac myosin light chain kinase is necessary for myosin regulatory light chain phosphorylation and cardiac performance in vivo." Journal of Biological Chemistry 285.52 (2010): 40819-40829.
[27] Huang, Jian, et al. "Myosin regulatory light chain phosphorylation attenuates cardiac hypertrophy." Journal of Biological Chemistry 283.28 (2008): 19748-19756.
[28] Davis, Julien S., et al. "The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation." Cell 107.5 (2001): 631-641.
[29] Tohtong, R., et al. "Dependence of metastatic cancer cell invasion on MLCK-catalyzed phosphorylation of myosin regulatory light chain." Prostate cancer and prostatic diseases 6.3 (2003): 212-216.
[30] Wu, Qian, et al. "Deficiency in myosin light-chain phosphorylation causes cytokinesis failure and multipolarity in cancer cells." Oncogene 29.29 (2010): 4183-4193.
[31] https://www.proteinatlas.org/ENSG00000101608-MYL12A/pathology
[32] Xiao, Xiao, et al. "Transformer with convolution and graph-node co-embedding: an accurate and interpretable vision backbone for predicting gene expressions from local histopathological image." Medical Image Analysis 91 (2024): 103040.
[33] Li, Yin-Chao, et al. "Oridonin suppress cell migration via regulation of nonmuscle myosin IIA." Cytotechnology 68 (2016): 389-397.
[34] Hosono, Yasuyuki, et al. "MYBPH inhibits NM IIA assembly via direct interaction with NMHC IIA and reduces cell motility." Biochemical and Biophysical Research Communications 428.1 (2012): 173-178.
[35] Du, Liwen, et al. "Inhibition of the MLCK/MLC2 pathway protects against intestinal heat stroke-induced injury in rats." Journal of Thermal Biology 116 (2023): 103655.
[36] Rath, Nicola, and Michael F. Olson. "Regulation of pancreatic cancer aggressiveness by stromal stiffening." Nature medicine 22.5 (2016): 462-463.
[37] Li, Laisi, et al. "Effects of CC-chemokine receptor 5 on ROCK2 and P-MLC2 expression after focal cerebral ischaemia–reperfusion injury in rats." Brain Injury 30.4 (2016): 468-473.
[38] Bright, Michael D., and Gad Frankel. "PAK4 phosphorylates myosin regulatory light chain and contributes to Fcγ receptor-mediated phagocytosis." The International Journal of Biochemistry & Cell Biology 43.12 (2011): 1776-1781.
[39] Oliveira, Andre M., and Margaret M. Chou. "The TRE17/USP6 oncogene: a riddle wrapped in a mystery inside an enigma." Frontiers in Bioscience-Scholar 4.1 (2012): 321-334.
[40] Oya, Ryohei, et al. "Phosphorylation of MYL12 by Myosin Light Chain Kinase Regulates Cellular Shape Changes in Cochlear Hair Cells." Journal of the Association for Research in Otolaryngology 22 (2021): 425-441.

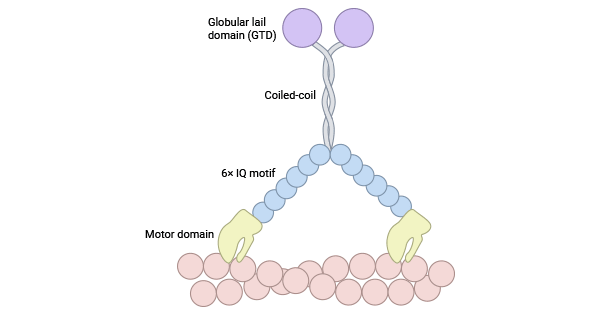
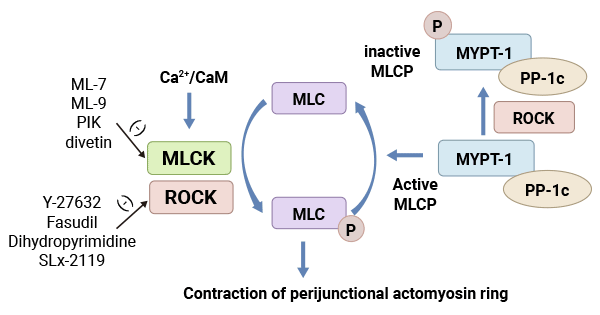
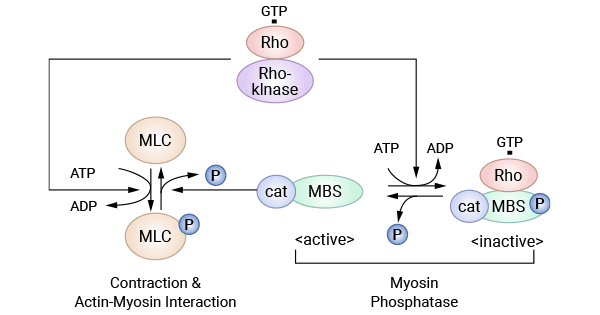
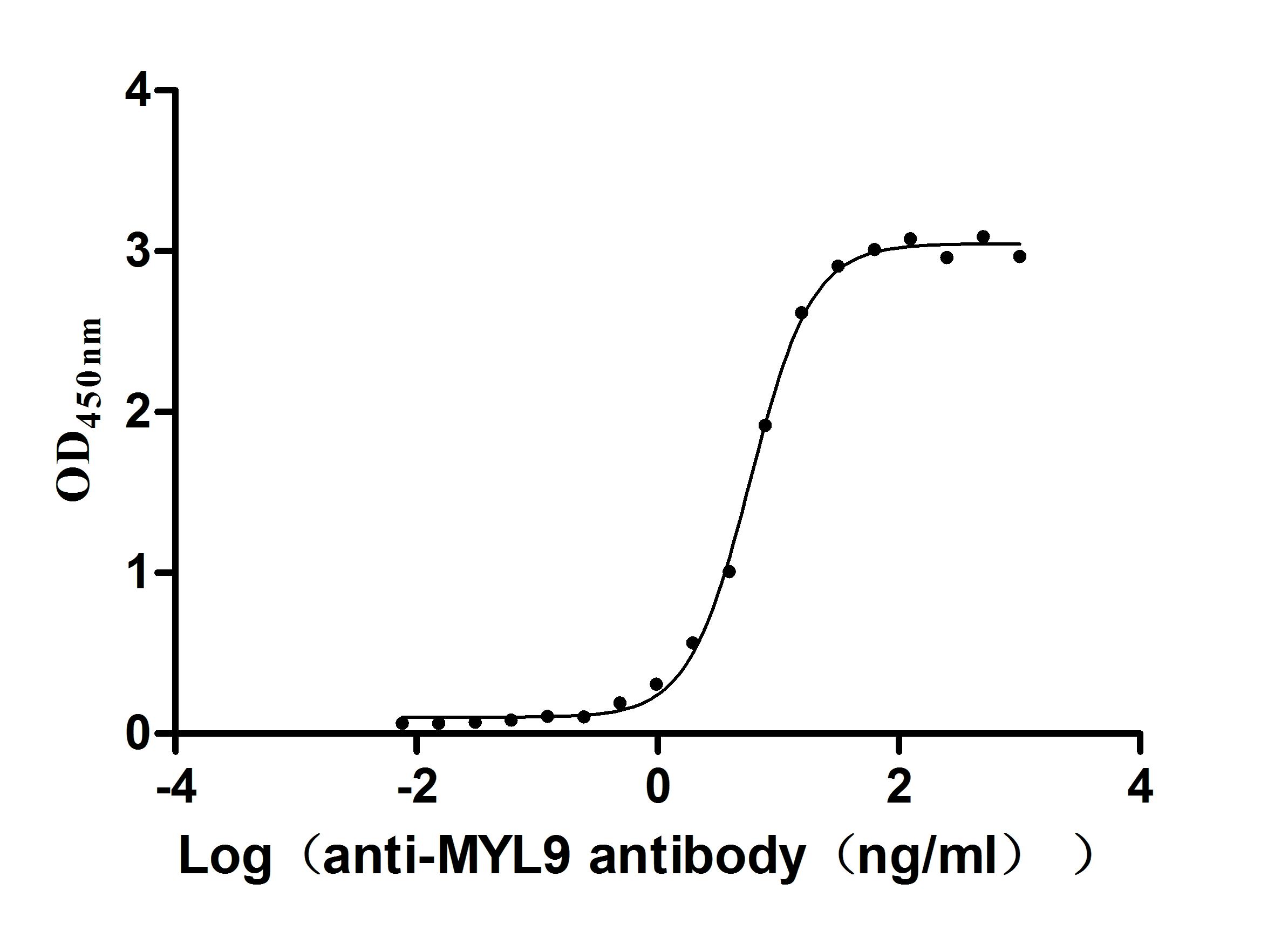
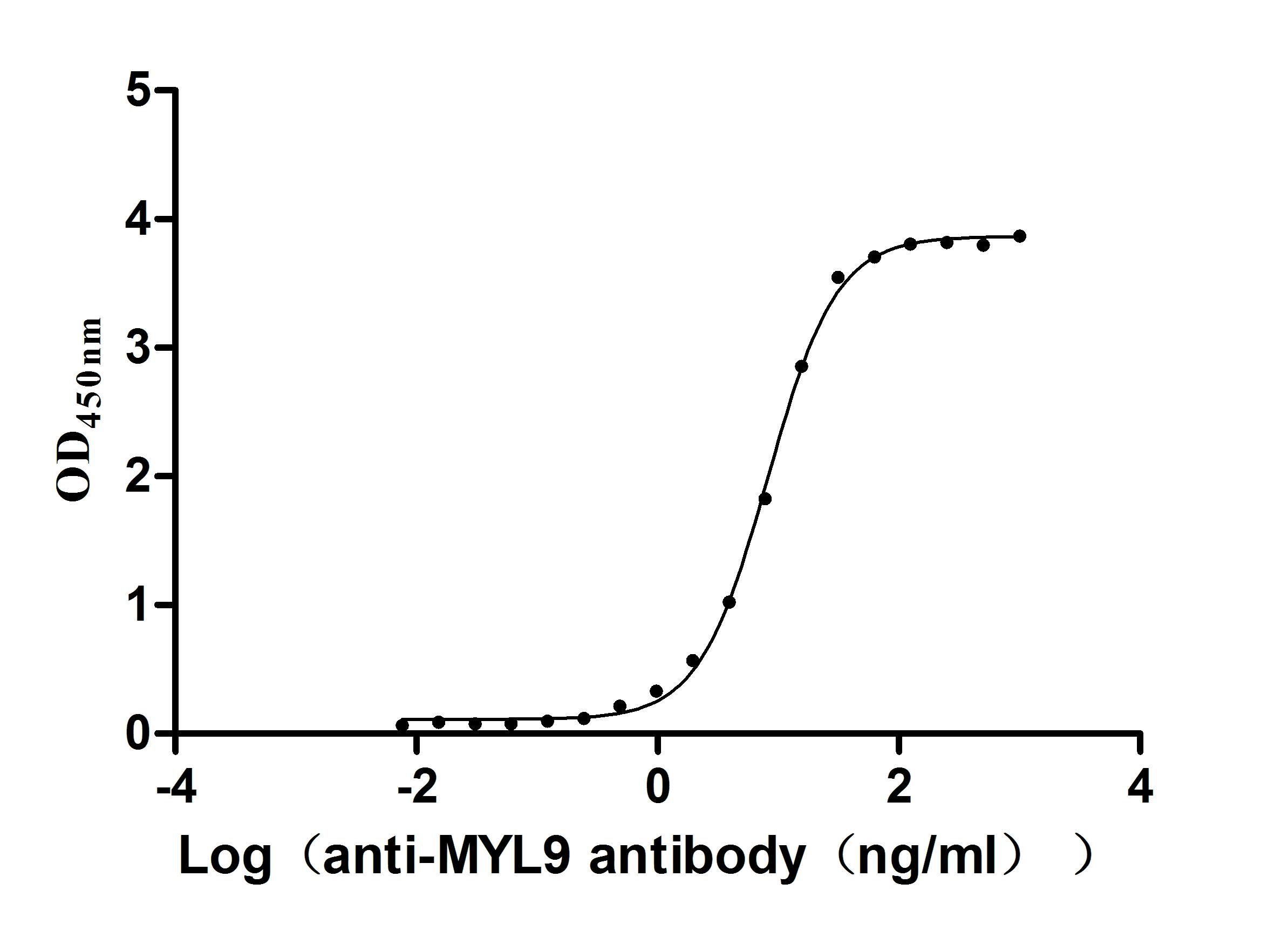


Comments
Leave a Comment