The process of generating recombinant human napsin-A (NAPSA) involves several critical steps, starting with isolating the target gene encoding the human NAPSA
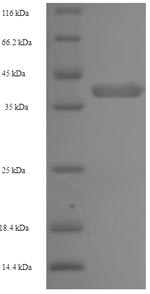
(64-420aa). This gene is co-cloned into an expression vector with an N-terminal 6xHis-tag gene and introduced into E. coli cells via transformation. The positive E. coli cells are selected and induced to express the recombinant NAPSA protein, which is harvested from the cell lysate. Purification of the recombinant NAPSA protein is typically achieved using affinity chromatography. The recombinant NAPSA protein's purity is analyzed by SDS-PAGE, exceeding 90%.
Napsin-A is an aspartic proteinase that plays a crucial role in various physiological processes. It is primarily expressed in the lung and kidney tissues [1][2]. In the lung, napsin-A is detected in normal type II pneumocytes, bronchiolar epithelial cells, and alveolar macrophages [3]. It is involved in the maturation of pro-surfactant protein B in type II pneumocytes and lysosomal protein catabolism in renal cells [4]. Evidence has shown that napsin-A is present in human lung tumors and inflammatory lung lesions, indicating its potential role in lung pathologies [1][2][5]. Furthermore, research has identified napsin-A as an immunohistochemical marker for renal cell carcinoma [6]. Its expression is found in the cytoplasm of pulmonary epithelial cells, renal proximal tubular epithelial cells, and various neoplasms [6].
References:
[1] T. Ueno, S. Linder, & G. Elmberger, Aspartic proteinase napsin is a useful marker for diagnosis of primary lung adenocarcinoma, British Journal of Cancer, vol. 88, no. 8, p. 1229-1233, 2003. https://doi.org/10.1038/sj.bjc.6600879
[2] J. Wu, P. Chu, Z. Jiang, & S. Lau, Napsin a expression in primary mucin-producing adenocarcinomas of the lung, American Journal of Clinical Pathology, vol. 139, no. 2, p. 160-166, 2013. https://doi.org/10.1309/ajcp62wjuamszcom
[3] I. Santos, J. Raiter, E. Lamego, M. Bandinelli, T. Pont, K. Siqueiraet al., Feline pulmonary carcinoma: gross, histological, metastatic, and immunohistochemical aspects, Veterinary Pathology, vol. 60, no. 1, p. 8-20, 2022. https://doi.org/10.1177/03009858221122517
[4] S. Weidemann, J. Böhle, H. Contreras, A. Luebke, M. Kluth, F. Büschecket al., Napsin a expression in human tumors and normal tissues, Pathology & Oncology Research, vol. 27, 2021. https://doi.org/10.3389/pore.2021.613099
[5] F. Berner, D. Bomze, C. Lichtensteiger, V. Walter, R. Niederer, O. Aliet al., Autoreactive napsin a–specific t cells are enriched in lung tumors and inflammatory lung lesions during immune checkpoint blockade, Science Immunology, vol. 7, no. 75, 2022. https://doi.org/10.1126/sciimmunol.abn9644
[6] T. Peat, E. Edmondson, M. Miller, D. DuSold, & J. Ramos‐Vara, Pax8, napsin a, and cd10 as immunohistochemical markers of canine renal cell carcinoma, Veterinary Pathology, vol. 54, no. 4, p. 588-594, 2017. https://doi.org/10.1177/0300985817698211
[9] M. Abdullah, First analysis of expression of napsin-a in ovine pulmonary adenomatosis of sheep and goat at duhok governorate, European Journal of Veterinary Medicine, vol. 3, no. 2, p. 19-23, 2023. https://doi.org/10.24018/ejvetmed.2023.3.2.86