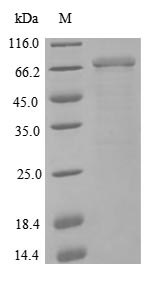
The gene fragment coding for the 31-677aa of the human NCAM1 protein is co-expressed with the N-terminal 6xHis tag gene in E.coli cells. An expression vector carries the target gene and is transfected into E.coli cells for protein expression. After cell lysis, the NCAM1 protein is purified using Ni-NTA affinity chromatography, exploiting the 6xHis tag for selective purification. The purified recombinant human NCAM1 protein is eluted with an imidazole gradient and analyzed using SDS-PAGE, showing a purity exceeding 90%.
The human NCAM1 is a significant member of the immunoglobulin superfamily, primarily recognized for its role in neural development and cellular interactions. NCAM1 is a glycoprotein that facilitates cell-cell and cell-matrix adhesion, which is crucial during various developmental processes, including neuronal migration, synaptogenesis, and neurite outgrowth [1][2]. This molecule is particularly important in the central nervous system, where it contributes to the structural integrity and functionality of neural tissues.
NCAM1 is involved in multiple biological processes, including cellular signaling, migration, proliferation, and differentiation. It acts as a signaling receptor that influences various cellular behaviors such as apoptosis and synaptic plasticity, which are vital for maintaining neuronal health and function [2][3]. The expression of NCAM1 has been linked to several human diseases, particularly in the context of tumors, where it is found to be upregulated in various malignancies, including nervous system tumors and lymphomas [2][4]. Its role in cancer is multifaceted, as NCAM1 not only promotes cell adhesion but also influences tumor cell migration and invasion, thereby affecting cancer progression and metastasis [3][5].
Moreover, NCAM1 is implicated in neurodevelopmental disorders and conditions such as schizophrenia, where its expression levels have been associated with disease severity and neuronal connectivity [6]. Genetic variations affecting NCAM1 have been identified as potential risk factors for congenital heart diseases, indicating its broader significance beyond the nervous system [7]. Additionally, NCAM1 has been shown to interact with polysialic acid, a modification that enhances its adhesive properties and is crucial for neuronal plasticity during development [8].
References:
[1] S. Sardana, A. Nederstigt, & M. Baggelaar, s-palmitoylation during retinoic acid-induced neuronal differentiation of sh-sy5y neuroblastoma cells, Journal of Proteome Research, vol. 22, no. 7, p. 2421-2435, 2023. https://doi.org/10.1021/acs.jproteome.3c00151
[2] R. Shukrun, H. Golan, R. Caspi, N. Pode‐Shakked, O. Pleniceanu, E. Vax, et al. Ncam1/fgf module serves as a putative pleuropulmonary blastoma therapeutic target, Oncogenesis, vol. 8, no. 9, 2019. https://doi.org/10.1038/s41389-019-0156-9
[3] L. Zhao, X. Wu, Z. Zhang, L. Fang, B. Yang, & Y. Li, Elf1 suppresses autophagy to reduce cisplatin resistance via the mir‐152‐3p/ncam1/erk axis in lung cancer cells, Cancer Science, vol. 114, no. 6, p. 2650-2663, 2023. https://doi.org/10.1111/cas.15770
[4] S. Zhong, B. Wu, J. Li, X. Wang, S. Jiang, F. Hu, et al. T5224, rspo2 and azd5363 are novel drugs against functional pituitary adenoma, Aging, vol. 11, no. 20, p. 9043-9059, 2019. https://doi.org/10.18632/aging.102372
[5] R. Takahashi, H. Ijichi, & M. Fujishiro, The role of neural signaling in the pancreatic cancer microenvironment, Cancers, vol. 14, no. 17, p. 4269, 2022. https://doi.org/10.3390/cancers14174269
[6] A. Gibbons, E. Thomas, & B. Dean, Regional and duration of illness differences in the alteration of ncam-180 mrna expression within the cortex of subjects with schizophrenia, Schizophrenia Research, vol. 112, no. 1-3, p. 65-71, 2009. https://doi.org/10.1016/j.schres.2009.04.002
[7] M. Yu, M. Aguirre, M. Jia, K. Gjoni, A. Córdova‐Palomera, C. Mungeret al., Oligogenic architecture of rare noncoding variants distinguishes 4 congenital heart disease phenotypes, Circulation Genomic and Precision Medicine, vol. 16, no. 3, p. 258-266, 2023. https://doi.org/10.1161/circgen.122.003968
[8] M. Langhauser, J. Ustinova, E. Rivera-Milla, D. Ivannikov, C. Seidl, C. Slomka, et al. Ncam1a and ncam1b: two carriers of polysialic acid with different functions in the developing zebrafish nervous system, Glycobiology, vol. 22, no. 2, p. 196-209, 2011. https://doi.org/10.1093/glycob/cwr129