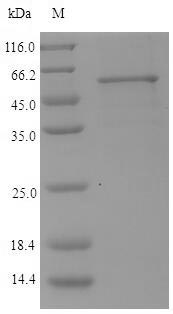
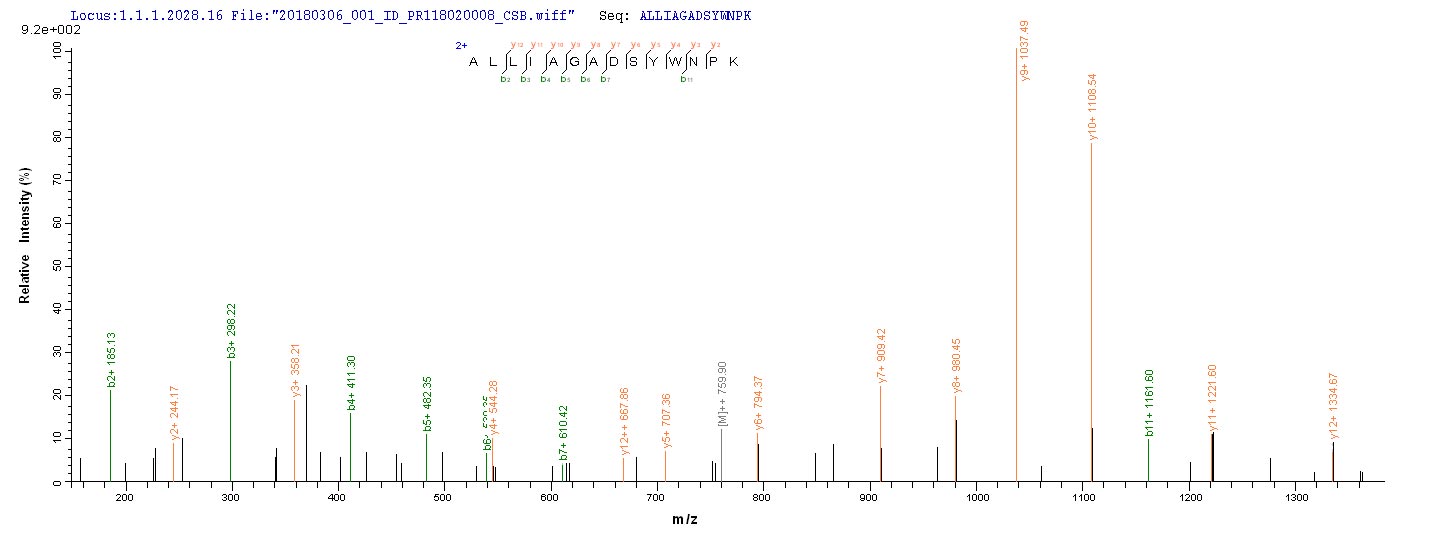
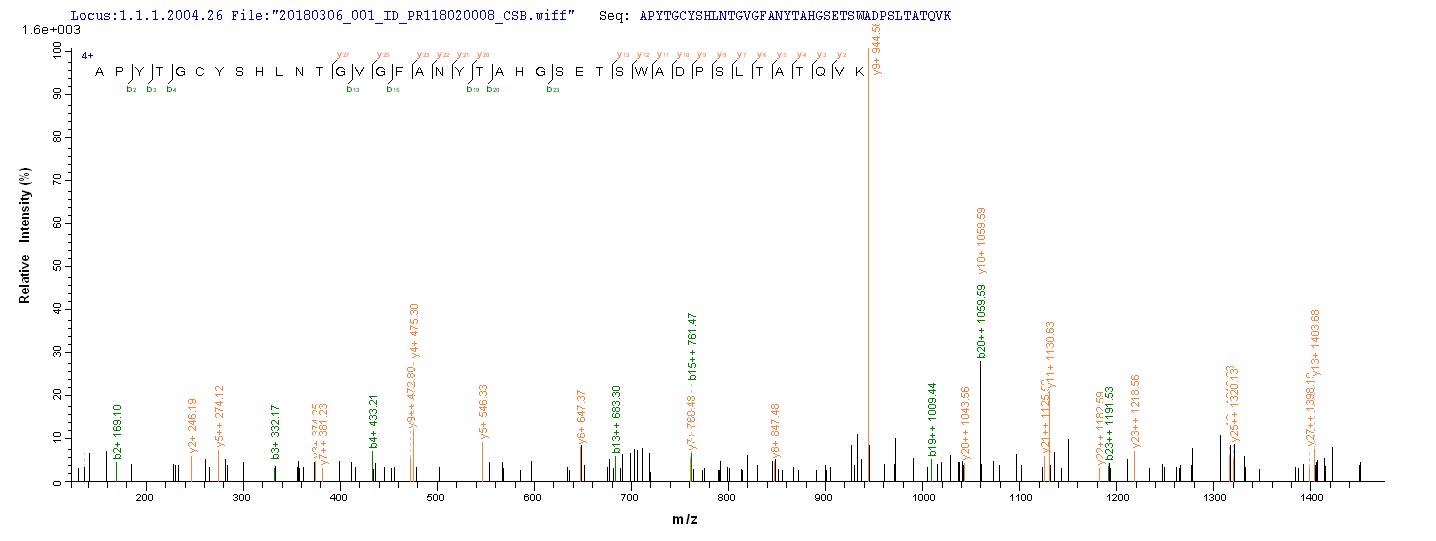
Lys-gingipain (Kgp) is a specific cysteine proteinase produced by Porphyromonas gingivalis, a bacterium associated with periodontal disease. Kgp is part of a group of proteases known as gingipains, which also include Arg-gingipains (Rgps). These gingipains play a crucial role in the virulence of P. gingivalis by aiding in the degradation of host tissues and evading the host immune response [1][2][3][4]. Kgp, along with Rgps, is involved in the pathogenesis of periodontal disease by hydrolyzing specific peptide bonds, with Kgp being specific for Lys-Xaa peptide bonds [4]. The secretion of Kgp is related to the cellular form and is essential for the bacterium's ability to interact with host cells and matrix proteins [5].
Studies have shown that P. gingivalis secretes significant amounts of Kgp on the cell surface and in the extracellular environment, highlighting the importance of this protease in the bacterium's pathogenicity [6]. Additionally, the activity of Kgp has been linked to hemoglobinase activity, indicating its role in nutrient acquisition and potentially in the modulation of the host environment to favor bacterial survival [7]. The presence and correct chromosomal location of the kgp gene, responsible for encoding Kgp, have been confirmed in P. gingivalis strains exhibiting Lys-gingipain activity [7].
References:
[1] J. Potempa, R. Pike, & J. Travis, "The multiple forms of trypsin-like activity present in various strains of porphyromonas gingivalis are due to the presence of either arg-gingipain or lys-gingipain", Infection and Immunity, vol. 63, no. 4, p. 1176-1182, 1995. https://doi.org/10.1128/iai.63.4.1176-1182.1995
[2] R. Takii, T. Kadowaki, A. Baba, T. Tsukuba, & K. Yamamoto, "A functional virulence complex composed of gingipains, adhesins, and lipopolysaccharide shows high affinity to host cells and matrix proteins and escapes recognition by host immune systems", Infection and Immunity, vol. 73, no. 2, p. 883-893, 2005. https://doi.org/10.1128/iai.73.2.883-893.2005
[3] E. Vanterpool, F. Roy, L. Sandberg, & H. Fletcher, "Altered gingipain maturation in vima- and vime-defective isogenic mutants of porphyromonas gingivalis", Infection and Immunity, vol. 73, no. 3, p. 1357-1366, 2005. https://doi.org/10.1128/iai.73.3.1357-1366.2005
[4] T. Imamura, "The role of gingipains in the pathogenesis of periodontal disease", Journal of Periodontology, vol. 74, no. 1, p. 111-118, 2003. https://doi.org/10.1902/jop.2003.74.1.111
[5] L. Wang, "Effects of orthodontic treatment on porphyromonas gingivalis, gingipains and gingival inflammation", European Journal of Inflammation, vol. 21, 2023. https://doi.org/10.1177/1721727x231220237
[6] K. Satô, E. Sakai, P. Veith, M. Shoji, Y. Kikuchi, H. Yukitakeet al., "Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of porphyromonas gingivalis", Journal of Biological Chemistry, vol. 280, no. 10, p. 8668-8677, 2005. https://doi.org/10.1074/jbc.m413544200
[7] J. Lewis, J. Dawson, J. Hannis, D. Muddiman, & F. Macrina, "Hemoglobinase activity of the lysine gingipain protease (kgp) of porphyromonas gingivalis w83", Journal of Bacteriology, vol. 181, no. 16, p. 4905-4913, 1999. https://doi.org/10.1128/jb.181.16.4905-4913.1999