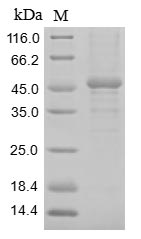
Recombinant Rat Heat-stable enterotoxin receptor (Gucy2c) is produced through a baculovirus expression system. The construct covers the extracellular domain spanning amino acids 23 to 429. For easier handling, the protein carries both an N-terminal 10xHis-tag and a C-terminal Myc-tag, which help with purification and detection steps. SDS-PAGE analysis shows the protein reaches greater than 85% purity, which appears sufficient for most biochemical assays and research work.
The Heat-stable enterotoxin receptor, Gucy2c, functions as a membrane-bound guanylate cyclase. This receptor seems to play an important role in how the intestinal epithelium manages fluid and electrolyte balance. Its involvement in maintaining intestinal homeostasis suggests it may be a central player in the cyclic GMP pathway. Researchers studying gastrointestinal function and related disorders have shown considerable interest in this protein.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
Rat Gucy2c is a complex transmembrane receptor whose extracellular domain requires precise folding, proper disulfide bond formation, and specific glycosylation patterns for ligand-binding activity. The baculovirus-insect cell expression system provides a eukaryotic environment that supports proper protein folding and some post-translational modifications. However, the extracellular domain fragment (23-429aa) lacks the transmembrane and intracellular domains that contribute to structural stabilization. The dual N-terminal 10xHis-tag and C-terminal Myc-tag may cause steric interference, particularly near potential ligand-binding regions. While the expression system increases folding probability, experimental validation is essential to confirm structural integrity and functional competence.
1. Protein-Protein Interaction Studies Using Pull-Down Assays
This application carries a significant risk due to the complex nature of receptor-ligand interactions. Gucy2c requires precise three-dimensional conformation for specific enterotoxin binding, which may be compromised by the dual-tag system and lack of transmembrane domain stabilization. The N-terminal His-tag may sterically block the ligand-binding pocket, while the C-terminal Myc-tag could interfere with receptor dimerization interfaces. Without confirmation of native folding through functional assays, pull-down experiments may yield false positives from non-specific binding or false negatives due to conformational defects.
2. Antibody Development and Characterization
This application is highly suitable because antibody generation primarily depends on linear epitope availability rather than functional protein conformation. The defined rat-specific sequence (23-429aa) of the extracellular domain serves as an excellent immunogen for generating antibodies that target the native, accessible region of the receptor. The high purity (>85%) minimizes immune responses to contaminants, while the dual tags facilitate efficient purification and screening. Antibodies developed against this immunogen will be valuable for detecting Gucy2c in various immunoassays regardless of the protein's functional status.
3. ELISA Development
This application is well-suited because ELISA relies on antigen-antibody binding rather than functional protein activity. This protein is well-suited for developing sandwich ELISA assays to detect or quantify the Gucy2c extracellular domain. The His-tag allows for direct and oriented coating onto Ni-NTA plates, while the Myc-tag provides a reliable epitope for detection antibodies, facilitating assay optimization. The protein can function effectively as a standard and a positive control. Its relevance is high for detecting soluble forms of the receptor or for antibody-sandwich assays. A key limitation is that it may not accurately reflect the exact conformation or glycosylation of the membrane-anchored, full-length receptor in vivo, which could affect the absolute quantitation of native Gucy2c from tissue samples.
4. Structural and Biochemical Characterization Studies
These studies are essential priority applications that provide critical quality control data about the protein itself, not the native Gucy2c. Size-exclusion chromatography can reveal oligomeric states and aggregation propensity, while circular dichroism spectroscopy assesses secondary structure content and thermal stability. Mass spectrometry can verify disulfide bond formation patterns crucial for proper folding. These biophysical analyses establish baseline parameters for comparing different protein batches and determining optimal storage conditions, forming the foundation for all subsequent functional studies.
Final Recommendation & Action Plan
The baculovirus expression system provides favorable eukaryotic folding conditions for this complex receptor domain, but the fragment nature and dual-tag configuration necessitate rigorous validation before functional applications. Implement a sequential validation protocol: begin with comprehensive biophysical characterization (Application 4) to assess structural integrity through SEC-MALS, CD spectroscopy, and disulfide bond analysis. Validate ligand-binding capability using known Gucy2c enterotoxins before proceeding to interaction studies (Application 1). Applications 2 and 3 (antibody development and ELISA) can proceed concurrently as they don't require functional validation. Always include appropriate controls: compare binding kinetics with tag-less constructs when possible, use native tissue samples for assay validation, and consider the tags' potential steric effects in data interpretation.