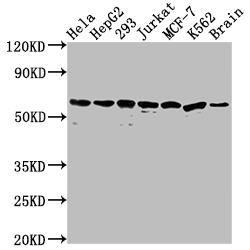
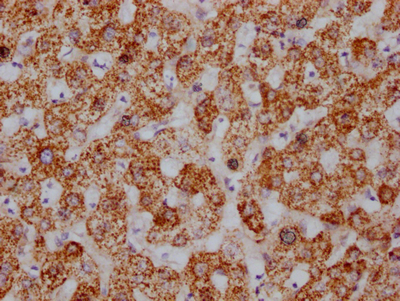
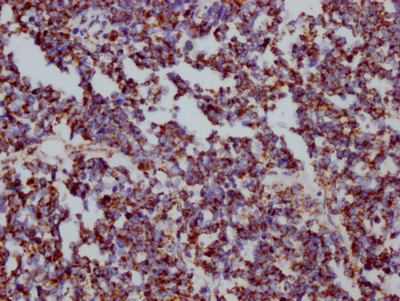
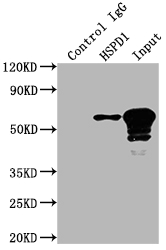
The HSPD1 recombinant monoclonal antibody is produced using protein and DNA recombinant technology. Initially, a synthesized peptide derived from human Hsp60 was used to immunize mice. After some time, the spleen of the mice was aseptically removed and the total RNA of spleen cells was extracted. The cDNA obtained from RNA reverse transcription served as the template for PCR amplification of the HSPD1 antibody gene. The HSPD1 antibody gene was then cloned into a vector, which was subsequently transfected into host cells for culture. The HSPD1 recombinant monoclonal antibody was purified from the cell culture supernatant using affinity chromatography. It has been rigorously tested and validated for its ability to detect human and mouse HSPD1 protein in ELISA, WB, and IHC, IP experiments.
The HSPD1 protein, also known as Hsp60, is a molecular chaperone that plays a key role in protein folding and assembly in the mitochondria of eukaryotic cells. It is involved in the folding and refolding of newly synthesized or denatured proteins. HSPD1 is a barrel-shaped protein complex that forms a cavity inside which unfolded or partially folded proteins can be properly folded in an ATP-dependent manner. In addition to its role in protein folding, HSPD1 has also been implicated in other cellular processes, including cell signaling, apoptosis, and immune response.