In the intricate world of pharmacokinetics, understanding how drugs move through the body is akin to unraveling a complex mystery. Compartment modeling is the key that unlocks this enigma, allowing researchers to visualize and predict the behavior of drugs as they journey through various physiological environments.
In this article, we will explore compartment modeling in pharmacokinetics and discuss its types, applications, advantages, and limitations.
Table of Contents
1. What Is Compartment Modeling in Pharmacokinetics?
Compartment modeling in pharmacokinetics is a mathematical approach that describes the absorption, distribution, metabolism, and excretion (ADME) of drugs within the body. This method simplifies the body's complexity by grouping tissues and fluids with similar pharmacokinetic properties into compartments. Each compartment represents a "virtual space" that approximates how a drug moves, allowing for better prediction of drug behavior based on various compartmental structures.
2. Types of Compartment Models
The most common compartment models include one-compartment, two-compartment, three-compartment, and physiologically-based pharmacokinetic (PBPK) models, each varying in complexity and applicability based on the drug's pharmacokinetic profile.
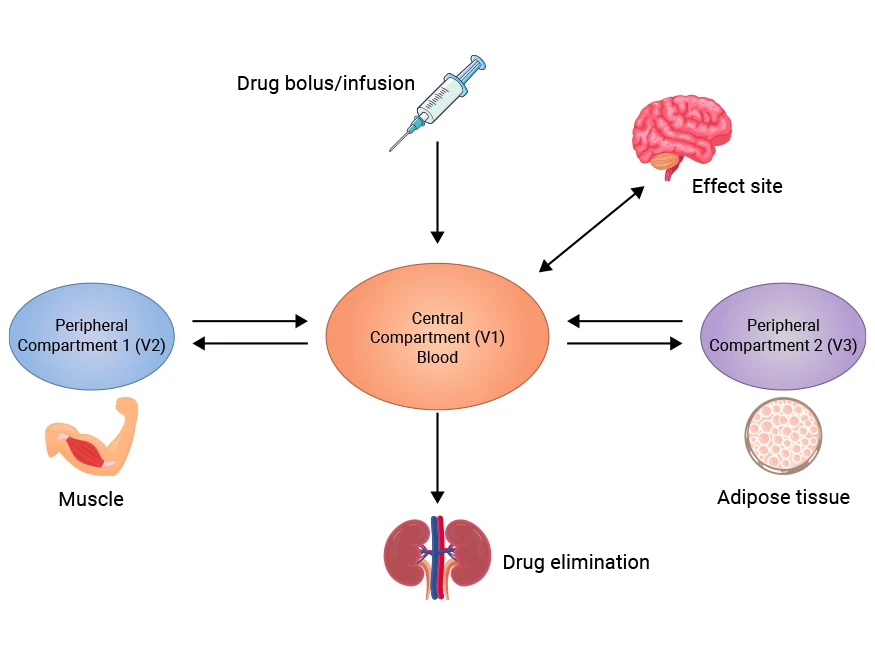
Figure 1. Three-compartment Model Diagram
This picture is cited from: https://www.sciencedirect.com/science/article/abs/pii/S1472029921002484
|
Compartment Model Types
|
Definition
|
Characteristics
|
Advantages
|
Disadvantages
|
Suitable Drug Types
|
|
One-Compartment Model
|
Assumes the body acts as a single, homogenous compartment where the drug is instantly and uniformly distributed.
|
-
Simplification: Assume that the drug is distributed uniformly in the body instantaneously.
-
Linearity: The drug elimination follows a first-order kinetics.
|
-
Easy to calculate: Due to its simple model, the calculation process is relatively easy.
-
Rapid application: Very effective for the preliminary evaluation of drug pharmacokinetic parameters
|
-
Not realistic enough: fails to take into account the different absorption and distribution of drugs by different tissues, and has limited applicability.
-
Narrow scope of application: Mainly suitable for drugs that are rapidly distributed and have no significant distribution phase in the body.
|
Fast-acting drugs, such as some antibiotics and analgesics.
|
|
Two-Compartment Model
|
Assumes that the body is divided into two main sections: the central compartment (blood and highly perfused organs such as the heart and liver) and the peripheral compartment (less-perfused tissues such as muscle and fat).
|
-
Distribution phase and elimination phase: contains a rapid distribution phase and a slower elimination phase.
-
More parameters: The drug distribution volume and elimination rate constant of the two compartments need to be estimated.
-
Linear: The distribution and elimination both follow first-order kinetics.
|
-
More accurate: It can better reflect the actual distribution of the drug in the body.
-
Wide applicability: Applicable to more types of drugs, especially those that take time to reach steady state after administration.
|
-
Increased complexity: The calculation and model establishment are relatively complex and require more parameter data.
-
High data requirements: Multiple sampling is required to accurately estimate the parameters.
|
Usually used for antiviral drugs, certain anti-tumor drugs and long-acting drugs.
|
|
Three-Compartment Model
|
Assumes that the body is divided into three section: the central compartment (the plasma), the peripheral compartment 1 (the highly perfused tissues), and the peripheral compartment 2 (the scarcely perfused tissues).
|
-
Subdivided distribution: including plasma compartment, peripheral tissue compartment, and deep tissue compartment.
-
More complex kinetics: describes the dynamic transfer of drugs between different tissues.
|
-
High accuracy: can simulate complex pharmacokinetic processes more accurately.
-
Adaptable to multiple situations: Suitable for situations where drugs have significant differences among multiple tissues.
|
-
Computational difficulties: Model construction and parameter estimation are complex because multiple chambers are involved.
-
Large data requirements: A large amount of experimental data is required to support the construction of the model.
|
Suitable for drugs that need to be distributed in multiple physiological environments.
|
|
PBPK Model
|
Considers the body as multiple interconnected chambers, each of which represents a different tissue or organ.
|
-
Physiologically driven: The model is built based on real physiological, anatomical and biochemical data.
-
Strong mechanism: The absorption, distribution, metabolism and excretion of drugs are described by a series of differential equations.
|
-
Highly flexible: It can simulate the pharmacokinetics of different individuals and under different conditions.
-
Widely used: Can be used for the development of new drugs, dose prediction and personalized treatment.
|
-
Complex model: Advanced mathematical and biological knowledge is required to build and verify the model.
-
High computing resource requirements: It requires strong computing power and a large amount of input data.
|
Suitable for situations where complex drugs and their metabolic pathways are unclear, especially when developing new drugs.
|
3. Why Compartment Models Are Designed in Pharmacokinetics?
Compartment models in pharmacokinetics are designed to simplify the complex processes of drug absorption, distribution, metabolism, and excretion (ADME) into manageable, quantifiable systems.
Compartment modeling not only provides a theoretical basis and tool support for drug research but also plays a key role in drug development and clinical application. By continuously improving and developing compartment models, researchers can better understand the behavior of drugs in the body and promote the development of new drugs and the realization of personalized treatment.
Commonly used in pharmacokinetics and clinical drug development, compartment models make it easier to determine how a drug should be dosed for optimal therapeutic effects while minimizing side effects. Compartment modeling also aids in the analysis of bioavailability, clearance, and the therapeutic index of drugs.
4. Advantages and Limitations of Compartment Modeling
Compartmental models have important application value in pharmacokinetics and can help scientists and doctors better understand the behavior of drugs in the body. However, they also have certain limitations, especially in terms of the accuracy of assumptions, biological variability, and computational complexity. Therefore, when using compartmental models, researchers should carefully consider these factors to ensure the reliability and applicability of model results.
Advantages
Simplicity and interpretability: Compartment models provide a simplified representation of complex pharmacokinetic processes, making it easier to predict drug concentration profiles and understand ADME behavior.
Quantitative analysis: This modeling method allows for quantitative analysis of the distribution of drugs in different compartments, allowing researchers to accurately estimate the concentration of drugs in the body and related pharmacokinetic parameters. This ability is very important in drug development and clinical treatment, especially in the study of the relationship between tumor characteristics and contrast agent distribution.
Explore individual differences: Through compartmental models, researchers can study pharmacokinetic variations caused by intrinsic factors (such as age, gender, genes, etc.). This exploration is crucial to the development of personalized medicine.
Comparison between multiple studies: Another advantage of the compartment model is that it enables comparison between multiple studies. With a unified model framework, researchers can more easily evaluate the behavior of drugs under different conditions, thereby improving the reproducibility and reliability of the results.
Time invariance: The linear, time-invariant compartment model provides a stable basis for the analysis of pharmacokinetics. This property enables the model to make effective predictions at different time points, which is crucial for the formulation of clinical trials and treatment plans.
Applicable to different routes of administration: The compartment model can adapt to a variety of administration methods such as oral, intravenous injection, etc., which makes it widely used in drug development and drug monitoring.
Limitations
Limitations of model assumptions: The compartment model is usually based on some assumptions, such as the uniform distribution of drugs in the body. In actual situations, the distribution of drugs may not be uniform, which may lead to reduced accuracy of the model. In particular, the one-compartment model has poor accuracy because it assumes that the drug is evenly distributed throughout the body.
Complexity of non-closed systems: Unlike closed system models, pharmacokinetic models do not return from output to input. This non-closed nature makes modeling and prediction more complicated because the direction and speed of drug flow can be affected by many factors.
Biological variability: Due to biological differences between individuals, the compartment model may not accurately predict the pharmacokinetic characteristics of each patient. For example, some patients may respond to the drug significantly differently than predicted by the model, which may affect the therapeutic effect and safety.
Computational complexity: As the number of compartments and model complexity increase, the computational requirements also increase. This may lead to higher computational burden and time costs in practical applications, especially when conducting large-scale clinical trials.
Dependence on data quality: The effectiveness of the compartment model often depends on the quality of the input data. If the data is inaccurate or incomplete, the results of the model may mislead the decision. Therefore, ensuring high-quality data collection is key to the successful application of the compartment model.
Limitations of dynamic changes: The compartment model usually assumes that the pharmacokinetic parameters are time-invariant, but in actual applications, the kinetic characteristics of the drug may change over time. Such dynamic changes may cause the model's predictions to fail, especially in long-term treatment.
5. Applications of Compartment Modeling in Pharmacokinetics
Compartmental models are widely used in pharmacokinetics, covering many aspects from drug development, and clinical trial design to personalized treatment. They not only improve the efficiency and accuracy of drug research but also provide important support for clinical practice.
Drug dosage optimization: Compartmental models can help researchers and clinicians accurately predict changes in drug concentration in the body. This prediction is crucial for adjusting drug dosage and evaluating therapeutic effects. By analyzing the distribution of drugs in different compartments, researchers can better understand the kinetic characteristics of drugs and optimize dosing regimens.
Clinical trial design: During drug development, compartmental models are used to design and analyze clinical trials. Researchers can use these models to evaluate the effects of different routes of administration, doses, and dosing frequencies on drug concentrations. This helps set reasonable clinical trial parameters, thereby improving the success rate of trials and the reliability of data.
Drug-drug interaction studies: Compartmental models can be used to study the interactions between different drugs. By simulating the actions of drugs in the body, researchers can predict the effects of a certain drug on the metabolism of another drug. This is especially important in polypharmacy, where drug interactions may lead to reduced efficacy or increased side effects.
Personalized drug therapy: Compartmental models also play an important role in personalized medicine. By analyzing the patient's physiological characteristics and drug metabolism capabilities, doctors can use these models to develop personalized dosing regimens. This approach can significantly improve treatment efficacy while reducing the risk of adverse reactions.
Drug safety assessment: In the early stages of drug development, compartmental models can be used to assess drug safety. Researchers can predict the potential toxicity of drugs in the body by simulating the metabolism and excretion process of drugs. This assessment helps to screen out candidate drugs with higher safety, thereby improving research and development efficiency.
Metabolic kinetics research: Compartmental models also have important applications in metabolic kinetics research. By establishing different metabolic compartments, researchers can deeply analyze the metabolic pathways of drugs in the body. This helps to identify metabolites and their biological activities, thereby providing a basis for drug design and improvement.
Drug development and approval: During drug development and regulatory approval, compartmental models provide necessary data support for the pharmacokinetic characteristics of drugs. Regulatory agencies such as the FDA often require detailed pharmacokinetic analysis to ensure the safety and efficacy of drugs. Compartmental models provide a scientific basis for meeting these requirements.
Conclusion
Compartment modeling serves as a vital tool in pharmacokinetics, transforming the complex interactions of drugs within the body into manageable and understandable components. By breaking down these interactions into distinct compartments, researchers can better predict drug behavior, enhance drug design, and improve patient outcomes. As our understanding of these models evolves, so too will our ability to tailor therapies that meet individual needs, paving the way for more effective and personalized medicine.
In addition to the compartment models in pharmacokinetics, the DXD (Exatecan derivative for ADC) is a highly potent payload for antibody-drug conjugates (ADCs). To thoroughly assist researchers and pharmaceutical companies in the pharmacokinetic analysis of DXD-ADC, CUSABIO has developed the PK research tool—DXD Monoclonal Antibody—which offers high specificity, stability, and sensitivity, allowing for effective determination of DXD's stability and release efficiency.
For more information, please read the article: Anti-Payload Antibody DXD: A Highly Potent Tool for ADCs Pharmacokinetic (PK) Analysis!
CUSABIO team. What Is Compartment Modelling in Pharmacokinetics?. https://www.cusabio.com/c-21197.html



Comments
Leave a Comment