[1] Andrew A. Laskin, Nikolai A. Kudryashov, Konstantin G. Skryabin, Eugene V. Korotkov.(2004). Latent periodicity of serine-threonine and tyrosine protein kinases and another protein families.
[2] Rabina Shrestha, Tess McCann, Harini Saravanan, Jaret Lieberth, Prashanna Koirala, Joshua Bloomekatz.(2023). The myocardium utilizes a platelet-derived growth factor receptor alpha (Pdgfra)–phosphoinositide 3-kinase (PI3K) signaling cascade to steer toward the midline during zebrafish heart tube formation.
[3] Katherine A. Fantauzzo, Philippe Soriano.(2014). PI3K-mediated PDGFRα signaling regulates survival and proliferation in skeletal development through p53-dependent intracellular pathways.
[4] Alisa A. Mueller, Cindy T. J. van Velthoven, K. Fukumoto, Tom H. Cheung, T. Rando.(2016). Intronic polyadenylation of PDGFRα in resident stem cells attenuates muscle fibrosis.
[5] Kim van Kuijk, Ian R McCracken, Renée J H A Tillie, Sebastiaan E J Asselberghs, Dlzar A Kheder, Stan Muitjens, Han Jin, Richard S Taylor, Ruud Wichers Schreur, Christoph Kuppe, Ross Dobie, Prakesh Ramachandran, Marion J Gijbels, Lieve Temmerman, Phoebe M Kirkwoord, Joris Luyten, Yanming Li, Heidi Noels, Pieter Goossens, John R Wilson-Kanamori, Leon J Schurgers, Ying H Shen, Barend M E Mees, Erik A L Biessen, Neil C Henderson, Rafael Kramann, Andrew H Baker, Judith C Sluimer.(2023). Human and murine fibroblast single-cell transcriptomics reveals fibroblast clusters are differentially affected by ageing and serum cholesterol.
[6] Elisa Manieri, Guodong Tie, Ermanno Malagola, Davide Seruggia, Shariq Madha, Adrianna Maglieri, Kun Huang, Yuko Fujiwara, Kevin Zhang, Stuart H Orkin, Timothy C Wang, Ruiyang He, Neil McCarthy, Ramesh A Shivdasani.(2023). Role of PDGFRA.
[7] Emilie Guérit, Florence Arts, Guillaume Dachy, Boutaina Boulouadnine, Jean-Baptiste Demoulin.(2021). PDGF receptor mutations in human diseases.
[8] Kentaro Mineji, Jun Watanabe, Eita Uchida, Savneet Kaur, Peng Zhang, Rintaro Hashizume.(2024). DDEL-03. INTRANASAL DELIVERY OF PDGFRA-TARGETED NANOTHERAPEUTICS FOR THE TREATMENT OF PEDIATRIC HIGH-GRADE GLIOMA.
[9] Quirinus J M Voorham, Beatriz Carvalho, Angela J Spiertz, Bart Claes, Sandra Mongera, Nicole C T van Grieken, Heike Grabsch, Martin Kliment, Bjorn Rembacken, Mark A van de Wiel, Philip Quirke, Chris J J Mulder, Diether Lambrechts, Manon van Engeland, Gerrit A Meijer.(2012). Comprehensive mutation analysis in colorectal flat adenomas.
[10] Yiting Deng, Yuanhang He, Juan Xu, Hao He, Manling Zhang, Guang Li.(2025). Cardiac fibroblasts regulate myocardium and coronary vasculature development in the murine heart via the collagen signaling pathway.
[11] T J MacDonald, K M Brown, B LaFleur, K Peterson, C Lawlor, Y Chen, R J Packer, P Cogen, D A Stephan.(2001). Expression profiling of medulloblastoma: PDGFRA and the RAS/MAPK pathway as therapeutic targets for metastatic disease.
[12] Jonathan Paolino, Boris Dimitrov, Beth Apsel Winger, Angelica Sandoval-Perez, Amith Vikram Rangarajan, Nicole Ocasio-Martinez, Harrison K Tsai, Yuting Li, Amanda L Robichaud, Delan Khalid, Charlie Hatton, Riaz Gillani, Petri Polonen, Anthony Dilig, Giacomo Gotti, Julia Kavanagh, Asmani A Adhav, Sean Gow, Jonathan Tsai, Yen Der Li, Benjamin L Ebert, Eliezer M Van Allen, Jacob Bledsoe, Annette S Kim, Sarah K Tasian, Stacy L Cooper, Todd M Cooper, Nobuko Hijiya, Maria Luisa Sulis, Neerav N Shukla, Jeffrey A Magee, Charles G Mullighan, Michael J Burke, Marlise R Luskin, Brenton G Mar, Matthew P Jacobson, Marian H Harris, Kimberly Stegmaier, Andrew E Place, Yana Pikman.(2023). Integration of Genomic Sequencing Drives Therapeutic Targeting of PDGFRA in T-Cell Acute Lymphoblastic Leukemia/Lymphoblastic Lymphoma.
[13] Michele Biscuola, Koen Van de Vijver, María Ángeles Castilla, Laura Romero-Pérez, María Ángeles López-García, Juan Díaz-Martín, Xavier Matias-Guiu, Esther Oliva, José Palacios Calvo.(2013). Oncogene alterations in endometrial carcinosarcomas.
[14] M E Brunkow, D L Nagle, A Bernstein, M Bucan.(1995). A 1.8-Mb YAC contig spanning three members of the receptor tyrosine kinase gene family (Pdgfra, Kit, and Flk1) on mouse chromosome 5.
[15] Carman K M Ip, Patrick K S Ng, Kang Jin Jeong, S H Shao, Zhenlin Ju, P G Leonard, Xu Hua, Christopher P Vellano, Richard Woessner, Nidhi Sahni, Kenneth L Scott, Gordon B Mills.(2018). Neomorphic PDGFRA extracellular domain driver mutations are resistant to PDGFRA targeted therapies.
[16] Qu-Jing Gai, Zhen Fu, Jiang He, Min Mao, Xiao-Xue Yao, Yan Qin, Xi Lan, Lin Zhang, Jing-Ya Miao, Yan-Xia Wang, Jiang Zhu, Fei-Cheng Yang, Hui-Min Lu, Ze-Xuan Yan, Fang-Lin Chen, Yu Shi, Yi-Fang Ping, You-Hong Cui, Xia Zhang, Xindong Liu, Xiao-Hong Yao, Sheng-Qing Lv, Xiu-Wu Bian, Yan Wang.(2022). EPHA2 mediates PDGFA activity and functions together with PDGFRA as prognostic marker and therapeutic target in glioblastoma.
[17] Thomas E Forman, Marcin P Sajek, Eric D Larson, Neelanjan Mukherjee, Katherine A Fantauzzo.(2024). PDGFRα signaling regulates Srsf3 transcript binding to affect PI3K signaling and endosomal trafficking.
[18] M. Riccetti, J. Green, Thomas J Taylor, A. Perl.(2023). Prenatal FGFR2 Signaling via PI3K/AKT Specifies the PDGFRA+ Myofibroblast.
[19] Xuehui Yang, Shivangi Pande, R. Koza, R. Friesel.(2021). Sprouty1 regulates gonadal white adipose tissue growth through a PDGFRα/β-Akt pathway.
[20] Chun-meng Wang, Ying-qiang Shi, Hong Fu, Guang-fa Zhao, Ye Zhou, Chun-yan Du, Yan-wei Ye.(2010). [Oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor].
[21] M J Ríos-Moreno, S Jaramillo, M Díaz-Delgado, M Sánchez-León, I Trigo-Sánchez, J Polo Padillo, J Amérigo, R González-Cámpora.(2011). Differential activation of MAPK and PI3K/AKT/mTOR pathways and IGF1R expression in gastrointestinal stromal tumors.
[22] Phillip Zook, H. Pathak, M. Belinsky, Lawrence Gersz, K. Devarajan, Yan Zhou, A. Godwin, M. von Mehren, L. Rink.(2016). Combination of Imatinib Mesylate and AKT Inhibitor Provides Synergistic Effects in Preclinical Study of Gastrointestinal Stromal Tumor.
[23] Martha L Slattery, Lila E Mullany, Lori C Sakoda, Roger K Wolff, John R Stevens, Wade S Samowitz, Jennifer S Herrick.(2018). The PI3K/AKT signaling pathway: Associations of miRNAs with dysregulated gene expression in colorectal cancer.
[24] Zhanghua Wu, Wei Zhao, Zhen Yang, Yue Wang, Yuichi Dai, Liang-an Chen.(2021). Novel Resistance Mechanisms to Osimertinib Analysed by Whole-Exome Sequencing in Non-Small Cell Lung Cancer.
[25] Kiran Kumar Chitluri, Emerson Isaac Arnold.(2025). Integrative genomic analysis identifies DPP4 inhibition as a modulator of FGF17 and PDGFRA downregulation and PI3K/Akt pathway suppression leading to apoptosis.
[26] Olga Martinho, R. Silva-Oliveira, Vera Miranda-Gonçalves, C. Clara, J. R. Almeida, A. Carvalho, J. Barata, R. Reis.(2013). In Vitro and In Vivo Analysis of RTK Inhibitor Efficacy and Identification of Its Novel Targets in Glioblastomas.
[27] Richard J Gilbertson, Jaqueline A Langdon, Andrew Hollander, Roberto Hernan, Twala L Hogg, Amar Gajjar, Christine Fuller, Steven C Clifford.(2006). Mutational analysis of PDGFR-RAS/MAPK pathway activation in childhood medulloblastoma.
[28] Olga Martinho, António Gouveia, Marta Viana-Pereira, Paula Silva, Amadeu Pimenta, Rui Manuel Reis, José Manuel Lopes.(2009). Low frequency of MAP kinase pathway alterations in KIT and PDGFRA wild-type GISTs.
[29] H. Cho, Junfei Zhao, S. Jung, Erik Ladewig, D. Kong, Y. Suh, Yeri Lee, Donggeon Kim, S. Ahn, M. Bordyuh, H. Kang, J. Sa, Y. Seo, S. Kim, D. Lim, Yun-Sik Dho, Jung-Il Lee, H. Seol, J. Choi, W. Park, Chul-Kee Park, R. Rabadán, D. Nam.(2018). Distinct genomic profile and specific targeted drug responses in adult cerebellar glioblastoma.
[30] Herbert M Sauro, Brian Ingalls.(2007). MAPK Cascades as Feedback Amplifiers.
[31] Paul Smolen, Douglas A. Baxter, John H. Byrne.(2008). Bistable MAP Kinase Activity: A Plausible Mechanism Contributing to Maintenance of Late Long-Term Potentiation.
[32] Ruth Nussinov, Chung-Jung Tsai, Carla Mattos.(2013). Pathway drug cocktail: targeting Ras signaling based on structural pathways.
[33] Joanne E Simpson, Noor Gammoh.(2024). Autophagy cooperates with PDGFRA to support oncogenic growth signaling.
[34] Lizhu Liu, Lihong Wu, D. Shan, Bo Han.(2022). Characterization and clinical relevance of PDGFRA pathway copy number variation gains across human cancers.
[35] Tamas Korcsmaros, Illes J. Farkas, Maté S. Szalay, Petra Rovo, David Fazekas, Zoltan Spiro, Csaba Bode, Katalin Lenti, Tibor Vellai, Peter Csermely.(2010). Uniformly curated signaling pathways reveal tissue-specific cross-talks and support drug target discovery.
[36] Cameron W Brennan, Roel G W Verhaak, Aaron McKenna, Benito Campos, Houtan Noushmehr, Sofie R Salama, Siyuan Zheng, Debyani Chakravarty, J Zachary Sanborn, Samuel H Berman, Rameen Beroukhim, Brady Bernard, Chang-Jiun Wu, Giannicola Genovese, Ilya Shmulevich, Jill Barnholtz-Sloan, Lihua Zou, Rahulsimham Vegesna, Sachet A Shukla, Giovanni Ciriello, W K Yung, Wei Zhang, Carrie Sougnez, Tom Mikkelsen, Kenneth Aldape, Darell D Bigner, Erwin G Van Meir, Michael Prados, Andrew Sloan, Keith L Black, Jennifer Eschbacher, Gaetano Finocchiaro, William Friedman, David W Andrews, Abhijit Guha, Mary Iacocca, Brian P O’Neill, Greg Foltz, Jerome Myers, Daniel J Weisenberger, Robert Penny, Raju Kucherlapati, Charles M Perou, D Neil Hayes, Richard Gibbs, Marco Marra, Gordon B Mills, Eric Lander, Paul Spellman, Richard Wilson, Chris Sander, John Weinstein, Matthew Meyerson, Stacey Gabriel, Peter W Laird, David Haussler, Gad Getz, Lynda Chin.(2013). The somatic genomic landscape of glioblastoma.
[37] H. Joensuu.(2023). KIT and PDGFRA Variants and the Survival of Patients with Gastrointestinal Stromal Tumor Treated with Adjuvant Imatinib.
[38] J. Dai, Y. Kong, L. Si, Z. Chi, C. Cui, X. Sheng, L. Mao, Si-ming Li, B. Lian, Ruifeng Yang, Shujing Liu, Xiaowei Xu, Jun Guo.(2013). Large-scale Analysis of PDGFRA Mutations in Melanomas and Evaluation of Their Sensitivity to Tyrosine Kinase Inhibitors Imatinib and Crenolanib.
[39] Tatsuya Kaji, Osamu Yamasaki, Minoru Takata, Masaki Otsuka, Toshihisa Hamada, Shin Morizane, Kenji Asagoe, Hiroyuki Yanai, Yoji Hirai, Hiroshi Umemura, Keiji Iwatsuki.(2017). Comparative study on driver mutations in primary and metastatic melanomas at a single Japanese institute: A clue for intra- and inter-tumor heterogeneity.
[40] Ryan A Denu, Cissimol P. Joseph, Elizabeth Urquiola, Precious S. Byrd, Richard K. Yang, R. Ratan, M. Zarzour, A. Conley, D. Araujo, V. Ravi, E. N. Nassif Haddad, M. Nakazawa, Shreyaskumar R Patel, Wei-Lien Wang, Alexander J. Lazar, N. Somaiah.(2024). Utility of Clinical Next Generation Sequencing Tests in KIT/PDGFRA/SDH Wild-Type Gastrointestinal Stromal Tumors.
[41] Changsong Qi, Fang Pan, Jian Li, Yanyan Li, Jing Gao, Lin Shen.(2018). [Analysis of biological characteristics and prognosis on gastrointestinal stromal tumor with PDGFRA gene mutation].
[42] Michael C Heinrich, Robert G Maki, Christopher L Corless, Cristina R Antonescu, Amy Harlow, Diana Griffith, Ajia Town, Arin McKinley, Wen-Bin Ou, Jonathan A Fletcher, Christopher D M Fletcher, Xin Huang, Darrel P Cohen, Charles M Baum, George D Demetri.(2008). Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor.
[43] C Serrano, S Bauer, D Gómez-Peregrina, Y-K Kang, R L Jones, P Rutkowski, O Mir, M C Heinrich, W D Tap, K Newberry, A Grassian, H Shi, S Bialick, P Schöffski, M A Pantaleo, M von Mehren, J C Trent, S George.(2023). Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib.
[44] D. Shepherd, T. Miller, D. Forst, P. Jones, V. Nardi, M. Martinez-Lage, A. Stemmer-Rachamimov, R. González, A. Iafrate, Lauren L. Ritterhouse.(2021). Mosaicism for receptor tyrosine kinase activation in a glioblastoma involving both PDGFRA amplification and NTRK2 fusion.
[45] Kohei Kanamori, Y. Yamagata, Y. Honma, Keiichi Date, T. Wada, Tsutomu Hayashi, Sho Otsuki, S. Sekine, T. Yoshikawa, H. Katai, T. Nishida.(2020). Extra-gastrointestinal stromal tumor arising in the lesser omentum with a platelet-derived growth factor receptor alpha (PDGFRA) mutation: a case report and literature review.
[46] Wen Huang, Wei Yuan, Lei Ren, Huaiyu Liang, Xiangyang Du, Xiangfei Sun, Yong Fang, Xiaodong Gao, Min Fu, Yihong Sun, Kuntang Shen, Yingyong Hou.(2022). Clinicopathological and therapeutic analysis of PDGFRA mutated gastrointestinal stromal tumor.
[47] Xuemeng Liu, Yaotian Hu, Z. Xue, Xun Zhang, Xiao-fei Liu, Guowei Liu, Muzi Wen, Anjing Chen, Bin Huang, Xia Li, Ning Yang, Jian Wang.(2023). Valtrate, an iridoid compound in Valeriana, elicits anti-glioblastoma activity through inhibition of the PDGFRA/MEK/ERK signaling pathway.
[48] Michael C Heinrich, Robin L Jones, Margaret von Mehren, Patrick Schöffski, César Serrano, Yoon-Koo Kang, Philippe A Cassier, Olivier Mir, Ferry Eskens, William D Tap, Piotr Rutkowski, Sant P Chawla, Jonathan Trent, Meera Tugnait, Erica K Evans, Tamieka Lauz, Teresa Zhou, Maria Roche, Beni B Wolf, Sebastian Bauer, Suzanne George.(2020). Avapritinib in advanced PDGFRA D842V-mutant gastrointestinal stromal tumour (NAVIGATOR): a multicentre, open-label, phase 1 trial.
[49] S. Abbou, C. Koschmann, C. Kramm, Lindsey M Hoffman, Ashley S. Plant-Fox, M. Abdelbaki, Ashley Bui, M. Casanova, Cornelis M. van Tilburg, Dong-Anh Khuong-Quang, Susan N Chi, Hongliang Shi, I. Bidollari, Janet Hong, P. Swamy, Maria Roche, D. Morgenstern.(2024). TRLS-08. ROVER: A PHASE 1/2 TRIAL IN PROGRESS OF AVAPRITINIB IN PEDIATRIC PATIENTS WITH SOLID TUMORS DEPENDENT ON KIT OR PDGFRA SIGNALING.
[50] R. Olivera-Salazar, Gabriel Salcedo Cabañas, L. Vega-Clemente, David Alonso-Martín, Víctor Manuel Castellano Megías, Peter Volward, D. García-Olmo, M. García-Arranz.(2024). Pilot Study by Liquid Biopsy in Gastrointestinal Stromal Tumors: Analysis of PDGFRA D842V Mutation and Hypermethylation of SEPT9 Presence by Digital Droplet PCR.
[51] Xue Kong, Jun Shi, Dongdong Sun, Lanqing Cheng, Can Wu, Zhiguo Jiang, Yushan Zheng, Wei Wang, Haibo Wu.(2025). A deep-learning model for predicting tyrosine kinase inhibitor response from histology in gastrointestinal stromal tumor.
[52] Pierre Noel.(2012). Eosinophilic myeloid disorders.
[53] C. Koschmann, L. Hoffman, C. Kramm, Ashley S. Plant-Fox, M. Abdelbaki, Ashley Bui, M. Casanova, D. Morgenstern, P. Swamy, Hongliang Shi, Janet Hong, Mikael L Rinne, S. Chi.(2023). TRLS-10. ROVER: A PHASE 1/2 STUDY OF AVAPRITINIB IN PEDIATRIC PATIENTS WITH SOLID TUMORS DEPENDENT ON KIT OR PDGFRA SIGNALING.
[54] Changgong Li, Matt K. Lee, F. Gao, S. Webster, H. Di, J. Duan, Chang-Yo Yang, Navin Bhopal, N. Peinado, G. Pryhuber, Susan M. Smith, Z. Borok, S. Bellusci, P. Minoo.(2019). Secondary crest myofibroblast PDGFRα controls the elastogenesis pathway via a secondary tier of signaling networks during alveologenesis.
[55] Celalettin Ustun, David L DeRemer, Cem Akin.(2011). Tyrosine kinase inhibitors in the treatment of systemic mastocytosis.
[56] Cameron Brennan, Hiroyuki Momota, Dolores Hambardzumyan, Tatsuya Ozawa, Adesh Tandon, Alicia Pedraza, Eric Holland.(2009). Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations.
[57] C. Cobbs, Sabeena Khan, Lisa Matlaf, Sean D McAllister, Alexander Zider, G. Yount, Kenneth Rahlin, L. Harkins, V. Bezrookove, Eric Singer, L. Soroceanu.(2014). HCMV glycoprotein B is expressed in primary glioblastomas and enhances growth and invasiveness via PDGFR-alpha activation.
[58] Soniya Bastola, Marat S Pavlyukov, Neel Sharma, Yasmin Ghochani, Mayu A Nakano, Sree Deepthi Muthukrishnan, Sang Yul Yu, Min Soo Kim, Alireza Sohrabi, Natalia P Biscola, Daisuke Yamashita, Ksenia S Anufrieva, Tatyana F Kovalenko, Grace Jung, Tomas Ganz, Beatrice O’Brien, Riki Kawaguchi, Yue Qin, Stephanie K Seidlits, Alma L Burlingame, Juan A Oses-Prieto, Leif A Havton, Steven A Goldman, Anita B Hjelmeland, Ichiro Nakano, Harley I Kornblum.(2025). Endothelial-secreted Endocan activates PDGFRA and regulates vascularity and spatial phenotype in glioblastoma.
[59] Joanne E Simpson, Morwenna T Muir, Martin Lee, Catherine Naughton, Nick Gilbert, Steven M Pollard, Noor Gammoh.(2024). Autophagy supports PDGFRA-dependent brain tumor development by enhancing oncogenic signaling.
[60] Xiaozhou Yu, Xiao Song, D. Tiek, Runxin Wu, Maya Walker, C. Horbinski, Bo Hu, Shi-Yuan Cheng.(2025). Abstract 6946: Targeting PDGFRa-SHP2 signaling enhances radiotherapy in IDH1 mutant glioma.
[61] Junya Yamaguchi, Fumiharu Ohka, Masafumi Seki, Kazuya Motomura, Shoichi Deguchi, Yoshiki Shiba, Yuka Okumura, Yuji Kibe, Hiroki Shimizu, Sachi Maeda, Yuhei Takido, Ryo Yamamoto, Akihiro Nakamura, Kennosuke Karube, Ryuta Saito.(2024). Dual phenotypes in recurrent astrocytoma, IDH-mutant; coexistence of IDH-mutant and IDH-wildtype components: a case report with genetic and epigenetic analysis.
[62] K. Tateishi, Yohei Miyake, Taishi Nakamura, Hiromichi Iwashita, Takahiro Hayashi, A. Oshima, Hirokuni Honma, Hiroaki Hayashi, Kyoka Sugino, Miyui Kato, K. Satomi, Satoshi Fujii, Takashi Komori, Tetsuya Yamamoto, D. Cahill, H. Wakimoto.(2023). Genetic alterations that deregulate RB and PDGFRA signaling pathways drive tumor progression in IDH2-mutant astrocytoma.

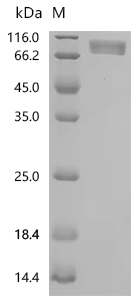
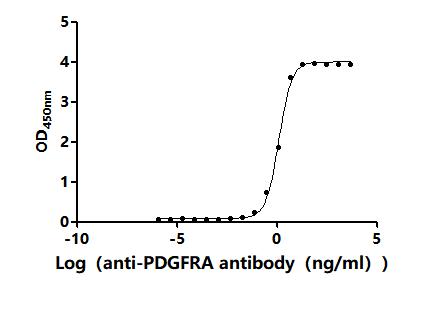
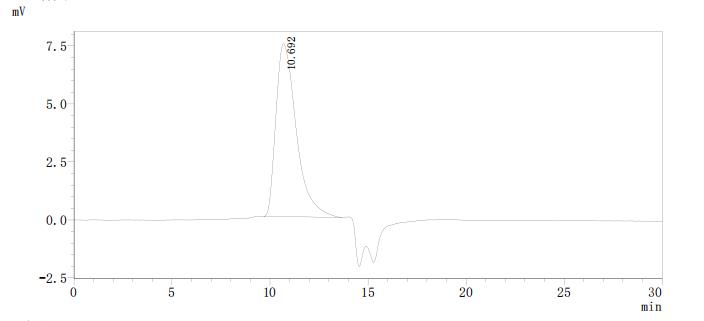
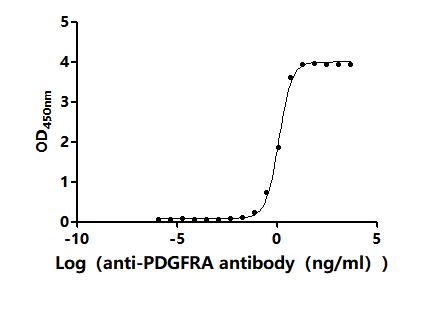



Comments
Leave a Comment