[1] Chou, C. W., Huang, Y. K., Kuo, T. T., Liu, J. P., & Sher, Y. P. (2020). An Overview of ADAM9: Structure, Activation, and Regulation in Human Diseases. International journal of molecular sciences, 21(20), 7790.
[2] Buranaphatthana, W., Wu, S., Makeudom, A., Sastraruji, T., Supanchart, C., & Krisanaprakornkit, S. (2021). Involvement of the A disintegrin and metalloproteinase 9 in oral cancer cell invasion.. European journal of oral sciences, e12775 .
[3] Zhou, R., Cho, W., , V., Cheuk, W., So, Y., Wong, S., Zhang, M., Li, C., Sun, Y., Zhang, H., Chan, L., & Tian, M. (2020). ADAM9 Mediates Triple-Negative Breast Cancer Progression via AKT/NF-κB Pathway. Frontiers in Medicine, 7.
[4] Oria, V., Lopatta, P., Schmitz, T., Preca, B., Nyström, A., Conrad, C., Bartsch, J., Kulemann, B., Hoeppner, J., Maurer, J., Bronsert, P., & Schilling, O. (2019). ADAM9 contributes to vascular invasion in pancreatic ductal adenocarcinoma. Molecular Oncology, 13, 456 - 479.
[5] Zhu, L., Zhao, Y., Yu, L., He, X., Wang, Y., Jiang, P., Yu, R., Li, W., Dong, B., Wang, X., & Dong, Y. (2021). Overexpression of ADAM9 decreases radiosensitivity of hepatocellular carcinoma cell by activating autophagy. Bioengineered, 12, 5516 - 5528.
[6] Moriwaki, M., Le, T., Sung, S., Jotatsu, Y., Yang, Y., Hirata, Y., Ishii, A., Chiang, Y., Chen, K., Shigemura, K., & Fujisawa, M. (2022). Relevance of A Disintegrin and Metalloproteinase Domain-Containing (ADAM)9 Protein Expression to Bladder Cancer Malignancy. Biomolecules, 12.
[7] Ueno, M., Shiomi, T., Mochizuki, S., Chijiiwa, M., Shimoda, M., Kanai, Y., Kataoka, F., Hirasawa, A., Susumu, N., Aoki, D., & Okada, Y. (2018). ADAM9 is over‐expressed in human ovarian clear cell carcinomas and suppresses cisplatin‐induced cell death. Cancer Science, 109, 471 - 482.
[8] Amêndola, R., Martin, A., Selistre-De-Araújo, H., Paula-Neto, H., Saldanha-Gama, R., & Barja-Fidalgo, C. (2015). ADAM9 disintegrin domain activates human neutrophils through an autocrine circuit involving integrins and CXCR2. Journal of Leukocyte Biology, 97.
[9] Arai, J., Otoyama, Y., Nozawa, H., Kato, N., & Yoshida, H. (2022). The immunological role of ADAMs in the field of gastroenterological chronic inflammatory diseases and cancers: a review. Oncogene, 42, 549 - 558.

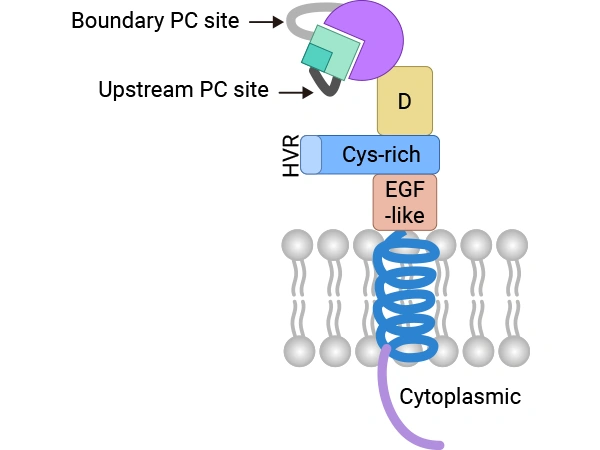
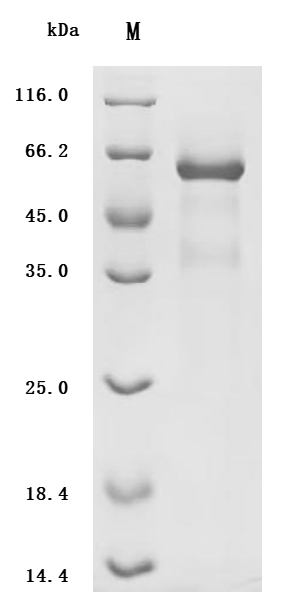
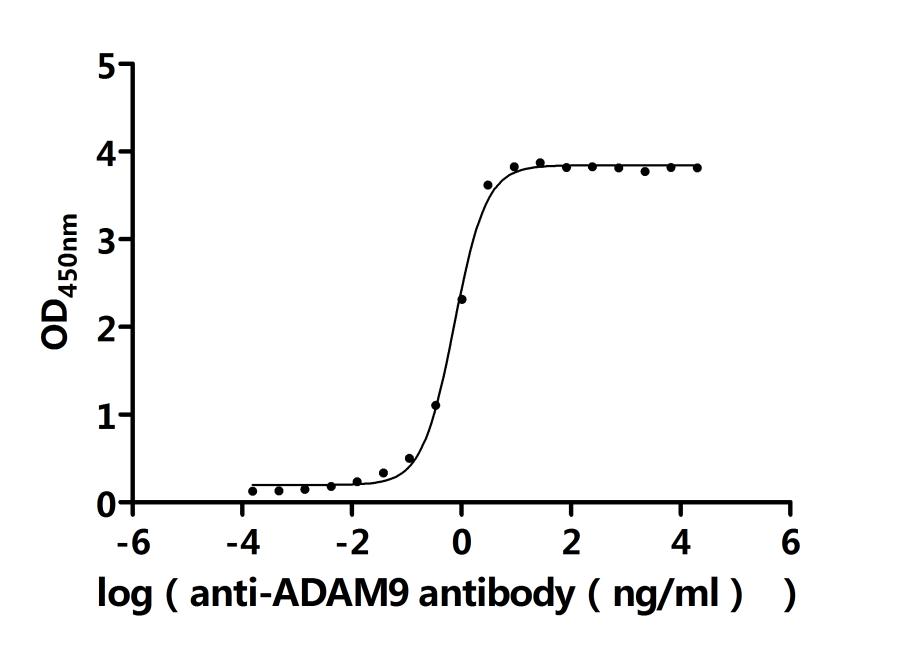

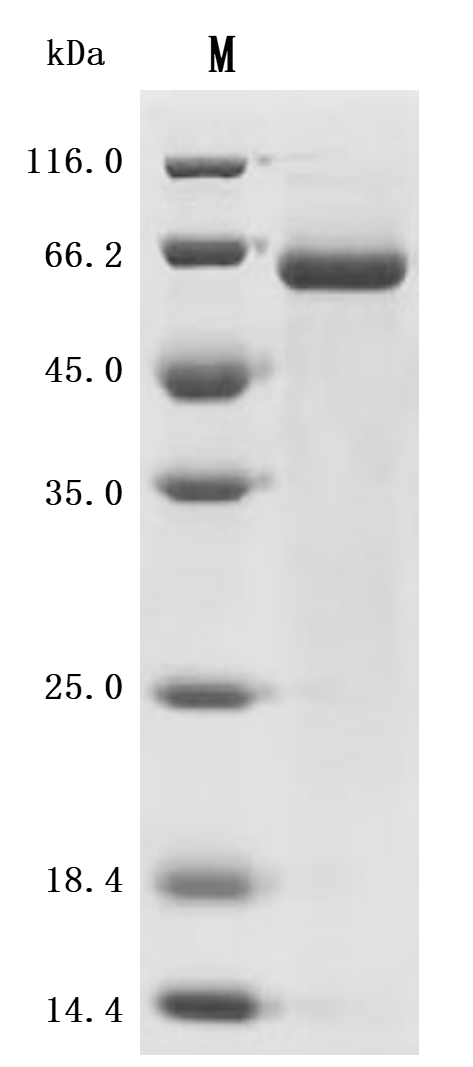
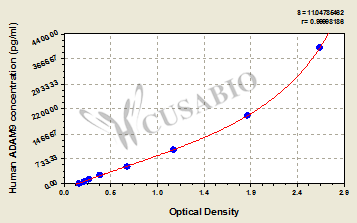



Comments
Leave a Comment