1. The Role and Importance of FCGR3A in the Immune System
Immunoglobulin G Fc segment receptor III A (FCGR3A, also known as CD16a) is a crucial activating receptor expressed on the surface of Natural Killer (NK) cells, monocytes, macrophages, and certain T cells. It belongs to the Fcγ receptor family [1].
By recognizing and binding to the Fc region of Immunoglobulin G (IgG), this receptor triggers intracellular signal transduction, regulating the activation, proliferation, and effector functions of immune cells. It plays a pivotal role in anti-infection, anti-tumor immunity, and autoimmune diseases. In recent years, with deepening research into immune checkpoints and the mechanisms of Antibody-Dependent Cellular Cytotoxicity (ADCC), the biological function and clinical value of FCGR3A as a significant target for immunotherapy have become increasingly prominent.
2. Molecular Structure, Expression Regulation, and Ligand Interaction
2.1 Structure of FCGR3A
FCGR3A belongs to the IgG Fc receptor family. The gene is located on the long arm of chromosome 1 (1q23) and encodes a type I transmembrane glycoprotein of approximately 50 - 65 kDa. It consists of two extracellular Ig-like domains, a single transmembrane region, and an intracellular tail.
Its intracellular region contains an Immunoreceptor Tyrosine-based Activation Motif (ITAM). By binding to the Fc region of IgG, it triggers a downstream signaling cascade, mediating Antibody-Dependent Cellular Cytotoxicity (ADCC) and Antibody-Dependent Cellular Phagocytosis (ADCP) [2].
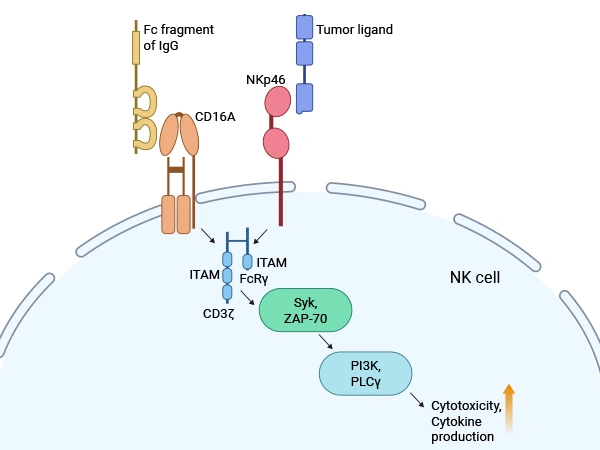
This figure elucidates the mechanisms by which CD16A and NKp46 receptors enhance NK cell functions.
Source: PMID: 39513028
The extracellular domain binds to the Fc region of IgG (especially IgG1 and IgG3 subclasses) with high affinity, triggering receptor cross-linking and signal transduction. Although the intracellular segment lacks intrinsic enzymatic activity, it recruits Syk or ZAP70 kinases via the ITAM, initiating downstream signaling cascades that promote cell activation, granzyme release, and cytokine secretion [3].
2.2 Expression and Regulation in Immune Cells
FCGR3A (CD16A) is primarily expressed in NK cells, subsets of monocytes/macrophages, and certain T cells. Its expression levels and functional regulation are closely related to immune responses.
NK Cells: FCGR3A is the key receptor mediating ADCC. Its surface density is influenced by genetic polymorphisms (such as FCGR3A-158V/F). The 158V allele is associated with higher receptor expression and ADCC activity.
Monocytes/Macrophages: Subsets with high FCGR3A expression are significantly enriched in kidney transplant rejection and the tumor microenvironment (TME), participating in antibody-mediated innate immune activation [4].
Regulation: FCGR3A expression is regulated by ADAM17-mediated proteolytic shedding, rapidly downregulating after leukocyte activation to limit excessive inflammatory responses [5].
T Cells: The CD8+CD11b+ subset can co-express FCGR3A and IL-2Rβ.
Tumor Microenvironment: High FCGR3A expression correlates with M2 macrophage infiltration and the upregulation of immune checkpoint molecules (such as PD-L1), suggesting a role in tumor immune escape.
2.3 Major Ligands
The core ligand of FCGR3A is the Fc region of IgG. Binding affinity is regulated by IgG glycosylation modifications (such as core fucosylation) and receptor polymorphisms.
Activation: Upon receptor cross-linking, phosphorylation of the ITAM motif recruits Syk kinase, activating the PLCγ, PI3K, and MAPK pathways. This promotes calcium influx, cytoskeletal rearrangement, and the release of effector molecules (Granzyme B, Perforin).
Synergy: FCGR3A signaling synergizes with Toll-like Receptor (TLR) or cytokine receptor signaling to enhance immune cell activation.
3. Signal Transduction and Immune Effector Mechanisms
3.1 Signal Transduction Pathways
ITAM-Dependent Activation: IgG binding → Src kinase-mediated ITAM phosphorylation → Syk kinase cascade → PI3K-Akt and PLCγ pathways.
PLCγ: Promotes IP3 production → Calcium influx → NFAT activation.
PI3K-Akt: Enhances cell metabolism and survival via the mTOR pathway.
Non-Canonical Regulation: In NK cells, FCGR3A synergizes with NKp46, amplifying signals through CD3ζ and FcRγ chains to promote cytokine release (IFN-γ, TNF-α) and cytotoxicity. In certain scenarios, the ITAM can exhibit a "dual nature" (ITAMi/ITAMa)—inhibiting signals upon monomeric binding but activating them upon multimeric cross-linking [6].
3.2 Immune Effector Mechanisms
Anti-Tumor Immunity: NK cells with high FCGR3A expression eliminate antibody-coated tumor cells via ADCC. Activity positively correlates with the high-affinity FCGR3A-V158 genotype. Bispecific antibodies (e.g., NK cell engagers) enhance killing by simultaneously targeting FCGR3A and tumor antigens (e.g., CD19).
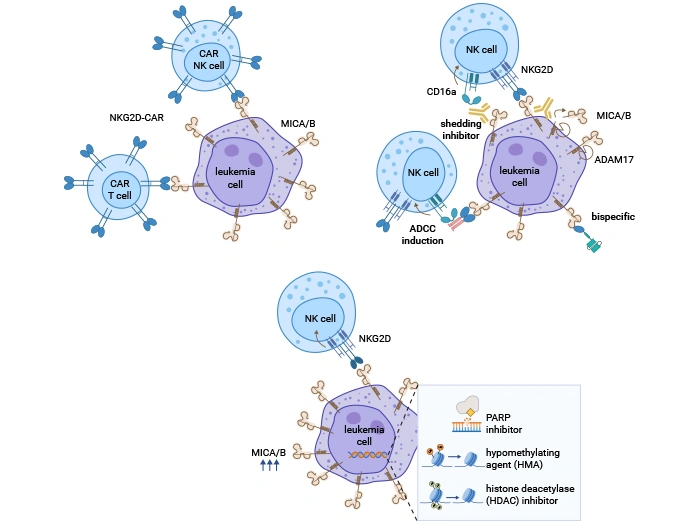
Modalities of NKG2DL-based immunotherapy in AML.
Source: PMID: 36555547
Anti-Infection Defense: Monocytes/macrophages clear IgG-opsonized pathogens (HIV, bacteria) via phagocytosis. IgG from HIV "Viral Controllers" shows stronger FCGR3A activation.
Inflammation: Excessive activation may lead to platelet aggregation (e.g., Heparin-Induced Thrombocytopenia) or tissue damage. High FCGR3A expression drives cytokine release (IL-12) in transplant rejection.
4. Role in Disease Pathogenesis
4.1 Autoimmune Diseases
Neuromyelitis Optica Spectrum Disorder (NMOSD): FCGR3A-FF genotype is associated with faster B-cell reconstitution and higher relapse rates; poorer response to rituximab.
Rheumatoid Arthritis (RA): FCGR3A-V carriers (VV/VF) show better response to rituximab and significant DAS28 score improvement.
Sjögren's Syndrome (SS): Activation upregulates glycolysis, promoting pro-inflammatory MDSC transformation and disrupting the Th17/Treg balance.
Inflammatory Bowel Disease (IBD): Drives M1 macrophage polarization; expression correlates with disease activity (AUC = 0.968).
4.2 Infectious Diseases
Dengue Fever: Cross-reactive antibodies may aggravate disease via Antibody-Dependent Enhancement (ADE).
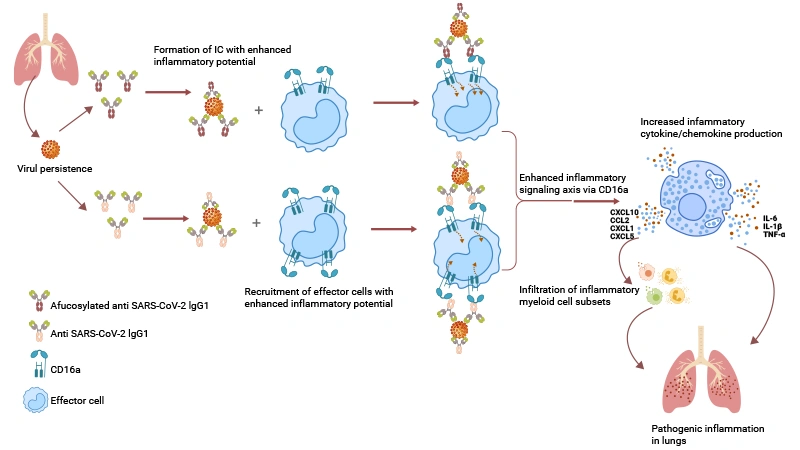
COVID-19: Enhanced CD16a:IgG signaling in severe patients promotes cytokine storms, possibly linked to afucosylated antibodies and persistent viral antigens.
Model for immunopathology of severe COVID‐19 mediated by enhanced CD16a:IgG signaling axis.
Source: PMID: 35781671
4.3 Tumor Microenvironment
Ovarian Cancer (HGSC): Specific "immune neighborhoods" of CD16a+ cells correlate with improved overall survival.
Solid Tumors: 158F allele carriers may have a better prognosis when treated with anti-EGFR antibodies (e.g., Cetuximab).
5. Progress in FCGR3A (CD16a) Targeted Drug Development
The landscape of FCGR3A-targeted therapies is diverse, including bispecific/trispecific NK cell engagers, CAR-NK, and antibodies.
Most Advanced: Clinical Phase 2 (e.g., Acimtamig for lymphoma).
Phase 1/2: Azerutamig for solid tumors.
Preclinical: Numerous candidates (e.g., TYRP1-TriNKET).
Selected Drug Pipeline:
|
Drug Name
|
Target (Gene Name)
|
Drug Type
|
Indications (Disease Name)
|
Institution / Organization
|
Highest Phase
|
|
Acimtamig
|
CD16a x CD30
|
Bispecific NK Cell Engager
|
CD30-Positive recurrent or refractory Cutaneous T-Cell Lymphoma |
Mycosis Fungoides | Peripheral T-cell Lymphoma | Relapsed B-cell
Lymphoma | Refractory B-cell Lymphoma | CD30+ Non-Hodgkin Lymphoma |
Refractory Peripheral T-cell Lymphoma | Relapsed Anaplastic Large Cell
Lymphoma | Refractory Classical Hodgkin Lymphoma | Refractory Anaplastic
Large Cell Lymphoma | Relapsed Classical Hodgkin Lymphoma
|
Affimed GmbH | The University of Texas MD Anderson Cancer Center
|
Phase 2
|
|
Azerutamig
|
CD16a x HER2 x NKG2D
|
Trispecific NK Cell Engager
|
Advanced Malignant Solid Tumors
|
Dragonfly Therapeutics, Inc.
|
Phase 1/2
|
|
SAR-445514
|
BCMA x CD16a x NKp46
|
CAR-NK
|
Multiple Myeloma | Tumors
|
Sanofi | Innate Pharma SA
|
Phase 1/2
|
|
IPH-6501
|
CD16a x CD20 x IL-2 x NKp46
|
NK Cell Engager | Tetraspecific Antibody
|
CD20-positive B-cell Non-Hodgkin Lymphoma | Diffuse Large B-Cell
Lymphoma (DLBCL) | Follicular Lymphoma | Mantle Cell Lymphoma |
Refractory Marginal Zone Lymphoma | Tumors
|
Innate Pharma SA
|
Phase 1/2
|
|
MUC1 x CD16A Bispecific antibody(BeiGene)
|
CD16a x MUC1
|
Bispecific NK Cell Engager
|
Advanced Cancer | Locally Advanced Malignant Solid Tumors | Metastatic
Solid Tumors | Metastatic Tumors | Solid Tumors | MUC1-Positive Solid
Tumors | Breast Cancer | Lung Cancer | Gastric Cancer
|
BeOne Medicines Ltd. | BeiGene Ltd.
|
Phase 1
|
|
AFM-28
|
CD123 x CD16a
|
Bispecific NK Cell Engager
|
Acute Myeloid Leukemia (AML) | Myelodysplastic Syndromes (MDS)
|
Affimed GmbH
|
Phase 1
|
|
SM3321
|
CD16a
|
Antibody
|
Locally Advanced Malignant Solid Tumors | Advanced Cancer |
Myelodysplastic Syndromes (MDS) | Acute Myeloid Leukemia (AML) |
Hematologic Malignancies
|
Zhuhai Sunbiotech Co., Ltd.
|
Phase 1
|
|
FT-576
|
BCMA x CD16a x IL-15Rα
|
CAR-NK | iPSC-derived
|
Refractory Multiple Myeloma | Relapsed Multiple Myeloma
|
Fate Therapeutics, Inc.
|
Phase 1
|
|
FT-596
|
CD16a x CD19 x IL-15Rα
|
CAR-NK | iPSC-derived
|
Relapsed B-cell Lymphoma | Refractory B-cell Lymphoma
|
University of Minnesota | Fate Therapeutics, Inc.
|
Phase 1
|
|
OXS-C3550
|
CD16a x CD33 x IL15R
|
Immunostimulatory Antibody Conjugate (ISAC)
|
Acute Myeloid Leukemia (AML) | Myelodysplastic Syndromes (MDS) | Myeloid
Tumors | Refractory Acute Myeloid Leukemia
|
University of Minnesota | GT Biopharma, Inc.
|
Phase 1
|
|
MK-4464
|
CD16a x CEACAM5 x NKG2D
|
Trispecific NK Cell Engager
|
Solid Tumors
|
Merck Sharp & Dohme Corp.
|
Phase 1
|
|
FT-538
|
CD16a x IL-15
|
NK Cell Therapy
|
Monocytic Leukemia | Refractory Acute Myeloid Leukemia
|
University of Minnesota Masonic Cancer Center
|
Phase 1
|
|
CIK(Ever Supreme Bio)
|
CD16a
|
Cytokine-Induced Killer Cell Therapy
|
Breast Cancer | Colorectal Cancer | Glioblastoma Multiforme | Liver
Cancer | Lung Cancer | Ovarian Epithelial Cancer | Pancreatic Cancer
|
Ever Supreme Bio Co., Ltd.
|
IND Approved (Clinical Trial Approved)
|
|
TYRP1-TriNKET
|
CD16a x NKG2D x TYRP1
|
Trispecific Antibody
|
Melanoma
|
Dragonfly Therapeutics, Inc.
|
Preclinical
|
|
BSI-111
|
CD16a
|
Monoclonal Antibody
|
Tumors
|
Biosion Inc.
|
Preclinical
|
|
AFM-32
|
CD16a x FOLR1
|
Fusion Protein
|
Solid Tumors
|
Affimed GmbH
|
Preclinical
|
|
NVS-32b
|
CD16a x CD32B
|
Monoclonal Antibody
|
Multiple Myeloma
|
Novartis Institutes for Biomedical Research, Inc.
|
Preclinical
|
|
CD19-targeted EVE16 memory-like NK cells
|
CD16a x CD19
|
NK Cell Therapy
|
Tumors
|
Inndura Therapeutics, Inc. | Massachusetts Institute of Technology |
Dana-Farber Cancer Institute, Inc.
|
Preclinical
|
|
CD16A (AI Proteins)
|
CD16a
|
Protein-based Drug
|
Tumors
|
AI Proteins, Inc.
|
Preclinical
|
|
SM2235
|
CD16a x EGFR
|
Bispecific Antibody
|
Solid Tumors
|
Zhuhai Sunbiotech Co., Ltd.
|
Preclinical
|
|
Anti-NKG2D/CD16/CEACAM5 Trispecific
|
CD16a x CEACAM5 x NKG2D
|
Trispecific Antibody
|
Tumors
|
Merck Sharp & Dohme LLC | Dragonfly Therapeutics, Inc.
|
Preclinical
|
|
FT-873
|
CD16a x CD276 x IL-7
|
Universal CAR-T
|
Solid Tumors
|
Fate Therapeutics, Inc.
|
Preclinical
|
|
FT-573
|
CD16a x CD276 x IL-15
|
Gene Therapy
|
Solid Tumors
|
Fate Therapeutics, Inc.
|
Preclinical
|
|
BiKE:HER2/CD16a
|
CD16a x HER2
|
Bispecific NK Cell Engager
|
Tumors
|
Rutgers State University of New Jersey
|
Preclinical
|
|
BiKE:E5C1
|
CD16a x HER2
|
Bispecific Antibody
|
HER2-Positive Breast Cancer
|
Rutgers State University of New Jersey
|
Preclinical
|
|
Trispecific NK cell engager
|
CD16a x EGFR x PDL1
|
Trispecific NK Cell Engager
|
Tumors
|
Technische Universit盲t Darmstadt | Ferring Holding SA
|
Preclinical
|
|
IPH-62
|
CD16a x CD276 x NKp46
|
Trispecific NK Cell Engager
|
Tumors
|
Innate Pharma SA | AstraZeneca PLC
|
Preclinical
|
|
Tri-modal CAR+TCR+hnCD16+iPSC-derived T cells
|
BCMA x CD16a x MICA x MICB x NY-ESO-1
|
iPSC-derived | CAR-T
|
Solid Tumors
|
Fate Therapeutics, Inc.
|
Preclinical
|
|
CD16/IL15/MSLN TriKE
|
CD16a x IL15R x MSLN
|
Trispecific NK Cell Engager
|
Non-Small Cell Lung Cancer (NSCLC)
|
University of Minnesota | GT Biopharma, Inc.
|
Preclinical
|
|
17C-02
|
CD16a
|
Monoclonal Antibody
|
Alloimmune Thrombocytopenia
|
Unity Health Toronto
|
Preclinical
|
|
iNK cells expressing CD64/16A
|
CD16a x CD64
|
NK Cell Therapy | iPSC-derived
|
Tumors
|
University of Minnesota
|
Preclinical
|
|
BCMA-CAR iT(Fate)
|
BCMA x CD16a x CD38
|
CAR-T
|
Multiple Myeloma
|
Fate Therapeutics, Inc.
|
Preclinical
|
|
CD16A/TAA BiKE (PersonGen)
|
CD16a x TAA
|
Bispecific NK Cell Engager
|
-
|
PersonGen BioTherapeutics (Suzhou) Co., Ltd.
|
Preclinical
|
|
cam1615TEM8
|
CD16a x IL15R x TEM8
|
Trispecific NK Cell Engager
|
Solid Tumors
|
University of Minnesota Twin Cities
|
Preclinical
|
|
CAM-161519
|
CD16a x CD19 x IL15R
|
Trispecific NK Cell Engager
|
Chronic Lymphocytic Leukemia (CLL)
|
GT Biopharma, Inc.
|
Preclinical
|
|
GTB-5550
|
CD16a x CD276 x IL-15
|
Trispecific NK Cell Engager
|
Head and Neck Cancer | Prostate Cancer | Head and Neck Squamous Cell
Carcinoma (HNSCC) | Multiple Myeloma
|
GT Biopharma, Inc. | Xcell Biosciences, Inc. | University of Minnesota
|
Preclinical
|
|
NAV-201
|
CD16a
|
Bispecific NK Cell Engager
|
Hematologic Malignancies | Solid Tumors
|
Navrogen, Inc.
|
Preclinical
|
|
BCMA x CD16 Dual Engagers
|
BCMA x CD16a
|
Bispecific NK Cell Engager
|
Multiple Myeloma
|
Oncopeptides AB
|
Preclinical
|
|
Anti-CD32B/CD16A Bispecific
|
CD16a x CD32B
|
Bispecific Antibody
|
Immune System Diseases | Tumors
|
MacroGenics, Inc.
|
Preclinical
|
|
Bi-Ab32/16
|
CD16a x HIV envelope protein gp120
|
双特异性抗体
|
HIV Infection
|
Autonomous University of Barcelona
|
Preclinical
|
|
PSMA TriKE
|
CD16a x IL-15R伪 x PSMA
|
Trispecific NK Cell Engager
|
Castration-Resistant Prostate Cancer (CRPC)
|
GT Biopharma, Inc.
|
Preclinical
|
|
IPH-64
|
BCMA x CD16a x NKp46
|
Trispecific NK Cell Engager
|
Tumors
|
Innate Pharma SA | Sanofi
|
Preclinical
|
|
AIP00000COC
|
CD16a x LILRB4
|
Trispecific NK Cell Engager
|
Acute Myeloid Leukemia (AML)
|
AI Proteins, Inc.
|
Preclinical
|
|
DF-4101
|
CD16a x NKG2D x c-Met
|
Trispecific NK Cell Engager
|
Tumors
|
Dragonfly Therapeutics, Inc.
|
Preclinical
|
|
MV-BiKE
|
CD16a x CEA
|
Bispecific NK Cell Engager
|
Tumors
|
University of Witten/Herdecke | German Cancer Research Center
|
Preclinical
|
|
Trispecific NK cell engager(Ferring Pharmaceuticals)
|
CD16a x EGFR x PDL1
|
Trispecific Antibody
|
Tumors
|
Ferring Holding SA
|
Preclinical
|
|
IBI3019
|
CD16a x CDH17 x EGFR
|
Trispecific Antibody
|
Colorectal Cancer
|
Innovent Biologics
|
Preclinical
|
|
MP0621
|
CD16a x CD47 x c-Kit
|
DARPin
|
Hematopoietic Stem Cell Transplantation | Acute Myeloid Leukemia (AML)
|
Molecular Partners AG
|
Preclinical
|
(Data as of November 20, 2025, sourced from Synapse)
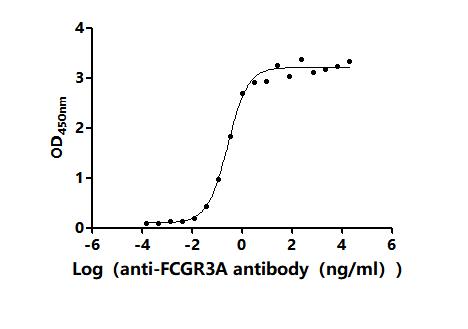
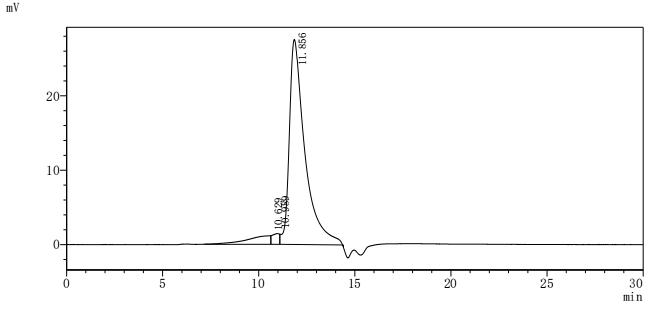
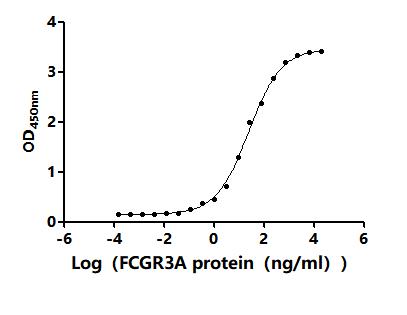
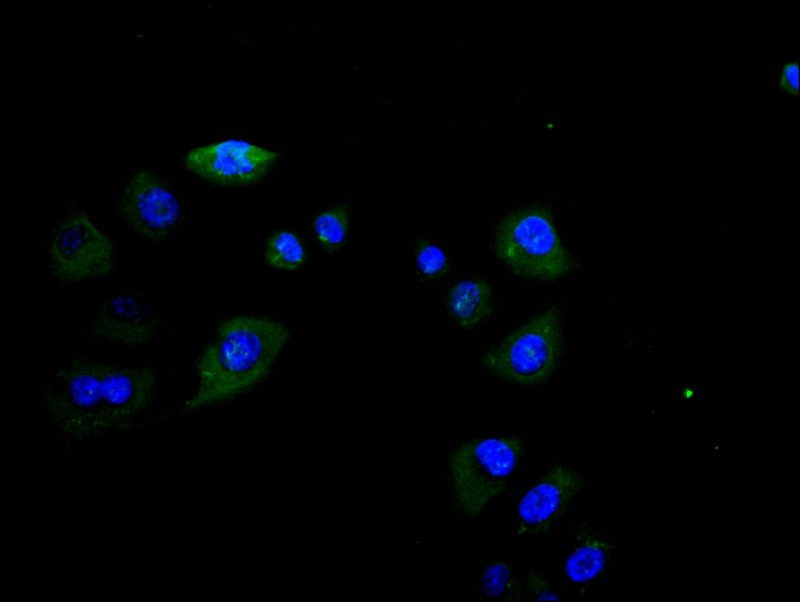
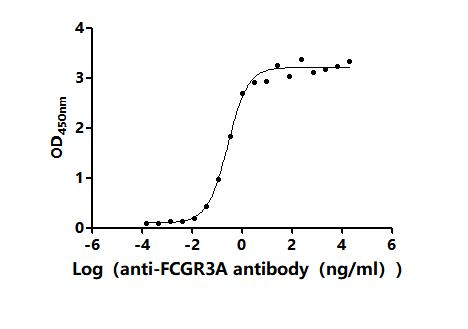
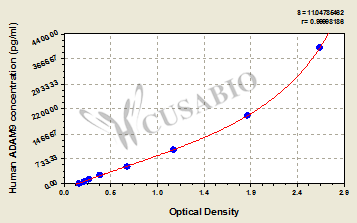
6. FCGR3A Research Tools
FCGR3A mechanisms are central to both innate and adaptive immunity. CUSABIO (Wuhan Huamei Biological) provides high-quality research tools to support mechanistic studies and drug development.
References
[1] Lampros, E. A. et al. The antibody-binding Fc gamma receptor IIIa/CD16a is N-glycosylated with high occupancy at all five sites. Curr. Res. Immunol. 2, (2022). https://doi.org/10.1016/j.crimmu.2022.05.005
[2] Kremer, P. G. et al. One N-glycan regulates natural killer cell antibody-dependent cell-mediated cytotoxicity and modulates Fc γ receptor IIIa/CD16a structure. eLife 13, (2024). https://doi.org/10.7554/eLife.100083
[3] Fogarty, C. A. et al. Oligomannose N-Glycans 3D architecture and its response to the FcγRIIIa structural landscape. J. Phys. Chem. B 125, (2021). https://doi.org/10.1021/acs.jpcb.1c00304
[4] Chen WongworawatYan et al. Spatial transcriptomics reveals distinct role of monocytes/macrophages with high FCGR3A expression in kidney transplant rejections. Front. Immunol. 16, (2025). https://doi.org/10.3389/fimmu.2025.1654741
[5] Hullsiek, R. et al. Examination of IgG Fc receptor CD16A and CD64 expression by canine leukocytes and their ADCC activity in engineered NK cells. Front. Immunol. 13, (2022). https://doi.org/10.3389/fimmu.2022.841859
[6] Ben Mkaddem, S. et al. Understanding Fc receptor involvement in inflammatory diseases: from mechanisms to new therapeutic tools. Front. Immunol. 10, (2019). https://doi.org/10.3389/fimmu.2019.00811
CUSABIO team. FCGR3A: A Key Receptor for Immune Regulation. https://www.cusabio.com/c-21282.html




Comments
Leave a Comment