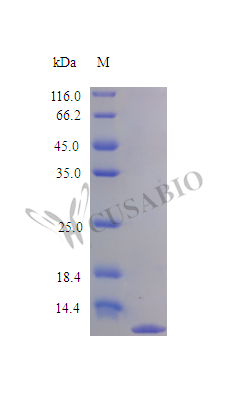
Recombinant Rat Eotaxin protein (Ccl11) is produced using an E. coli expression system, achieving purity levels exceeding 96% as confirmed by SDS-PAGE analysis. This tag-free protein represents the complete mature protein sequence, spanning residues 24 to 97. The protein demonstrates biological activity and shows efficacy in chemotaxis bioassays with purified eosinophils within a concentration range of 0.1-1.0 μg/ml. Endotoxin levels remain below 1.0 EU/μg, as determined by the LAL method.
Eotaxin, also known as Ccl11, functions as a chemokine that appears critical for recruiting eosinophils—specialized white blood cells that participate in immune responses, particularly during allergic and asthmatic reactions. It likely plays a significant role in directing eosinophil migration to inflammatory sites, which makes it an important research target for studying immune system function and inflammatory diseases.
Potential Applications
Note: The applications listed below are based on what we know about this protein's biological functions, published research, and experience from experts in the field. However, we haven't fully tested all of these applications ourselves yet. We'd recommend running some preliminary tests first to make sure they work for your specific research goals.
1. Eosinophil Chemotaxis Assays
This recombinant rat eotaxin (Ccl11) protein is confirmed to be biologically active in eosinophil chemotaxis (active at 0.1-1.0 μg/ml) and suitable as a positive control. However, the relatively high concentration range (100-1000 ng/ml) suggests moderate potency compared to some chemokines. Researchers should perform careful dose optimization for their specific eosinophil sources and assay conditions. The high purity (>96%) and low endotoxin levels support reliable results, but the effective concentration range indicates this protein may have a lower affinity for eosinophil receptors than typical chemokine-receptor interactions.
2. Comparative Species Studies of Chemokine Function
The protein enables valid comparative studies, but the high effective concentration range may indicate species-specific differences in receptor affinity. When comparing rat eotaxin with human or mouse versions, researchers should account for potential differences in potency and receptor binding characteristics. The tag-free design supports authentic comparisons, but parallel assays in the same experimental system are essential for accurate species comparisons.
3. Antibody Development and Validation
This high-purity, full-length mature eotaxin (24-97aa) serves as a good antigen for antibody development. However, antibodies should be validated against native rat eotaxin from biological sources to ensure recognition of physiologically relevant forms. The confirmed biological activity indicates proper folding, but the moderate potency (high working concentrations) may affect the sensitivity of functional antibody neutralization assays.
4. Receptor Binding and Competition Studies
The biologically active eotaxin is suitable for CCR3 binding studies, but the high effective concentration range (0.1-1.0 μg/ml) suggests moderate receptor affinity. Binding assays should be designed with appropriate concentration ranges to accurately determine kinetic parameters. Competition studies may require higher competitor concentrations than typically used for high-affinity chemokine-receptor interactions.
5. In Vitro Eosinophil Activation Studies
This eotaxin can be used for eosinophil activation studies, but the high working concentrations may cause non-specific effects in complex functional assays beyond chemotaxis. Researchers should include appropriate controls to distinguish specific eotaxin-mediated activation from potential non-specific stimulation at higher concentrations. Dose-response relationships should be carefully established for each activation parameter measured.
Final Recommendation & Action Plan
This recombinant rat eotaxin (Ccl11) is a validated tool for studying eosinophil biology, though its relatively high effective concentration range (0.1-1.0 μg/ml) indicates moderate potency compared to some chemokines. For immediate use, perform careful dose optimization in each specific assay system, as eosinophil sensitivity may vary by source and activation state. The high purity and low endotoxin make it suitable for sensitive cell-based assays, but researchers should include appropriate controls at higher concentrations to account for potential non-specific effects. When developing antibodies, validate against native eotaxin from allergic inflammation models to ensure biological relevance. For comparative studies, the species-specific sequence enables direct comparisons, but researchers should account for the moderate potency when interpreting functional differences between species. The E. coli expression produces a non-glycosylated protein, which may affect some functions but appears sufficient for core CCR3 engagement. Always include proper controls and consider that different eosinophil preparations may exhibit varying sensitivity to eotaxin stimulation.