[1] Yang, M., Wei, Z., Zhou, X., et al. (2020). A Fatal Case of MSTN Mutation Calf Pancreatitis. Preprints.
[2] Maeta, K., Farea, M., Nishio, H., & Matsuo, M. (2023). A novel splice variant of the human MSTN gene encodes a myostatin-specific myostatin inhibitor. Journal of Cachexia, Sarcopenia and Muscle, 14, 2289-2300.
[3] Campbell, C., et al. (2017). Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: Results of a randomized, placebo-controlled clinical trial. Muscle & Nerve, 55(4), 458-464.
[4] Grobet, L., Martin, L. J. R., Poncelet, D., et al. (1997). A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nature Genetics, 17(1), 71-74.
[5] McPherron, A. C., Lawler, A. M., & Lee, S. J. (1997). Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature, 387(6628), 83-90.
[6] Guo, R., Wang, H., Meng, C., et al. (2023). Efficient and Specific Generation of MSTN-Edited Hu Sheep Using C-CRISPR. Genes, 14(6), 1216.
[7] Suh, J., Kim, N. K., Lee, S. H., et al. (2020). GDF11 promotes osteogenesis as opposed to MSTN, and follistatin, a MSTN/GDF11 inhibitor, increases muscle mass but weakens bone. Proceedings of the National Academy of Sciences, 117(9), 4910-4920.
[8] Schuelke, M., Wagner, K. R., Stolz, L. E., et al. (2004). Myostatin mutation associated with gross muscle hypertrophy in a child. New England Journal of Medicine, 350(26), 2682-2688.
[9] Li, R., Zeng, W., Ma, M., et al. (2020). Precise editing of myostatin signal peptide by CRISPR/Cas9 increases the muscle mass of Liang Guang Small Spotted pigs. Transgenic Research, 29(2), 149-163.
[10] Wang, X., Niu, Y., Zhou, J., et al. (2016). Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Scientific Reports, 6, 32271.
[11] McPherron, A. C., & Lee, S. J. (1997). Double muscling in cattle due to mutations in the myostatin gene. Proceedings of the National Academy of Sciences, 94(23), 12457-12461.
[12] Eom, K.-H., Kwon, D.-H., Kim, Y.-C., et al. (2024). Novel Mammalian Ubiquitous Promoter Isolated from Bovine MSTN Gene Promoter. Preprints.
[13] Zhang, C., Liu, Y., Xu, D., et al. (2011). Polymorphisms of myostatin gene (MSTN) in four goat breeds and their effects on Boer goat growth performance. Molecular Biology Reports, 39(3), 3081-3087.
[14] Li, R., Zeng, W., Ma, M., et al. (2020). CRISPR/Cas9-mediated MSTN disruption accelerates the growth of Chinese Bama pigs. Reproduction in Domestic Animals, 55(6), 1314-1327.

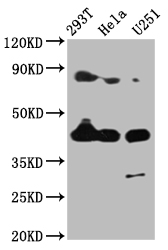
-SDS.jpg)
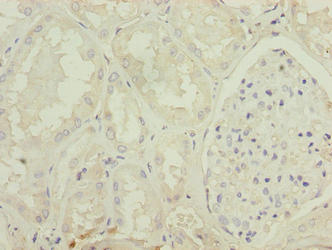
-AC1.jpg)


Comments
Leave a Comment