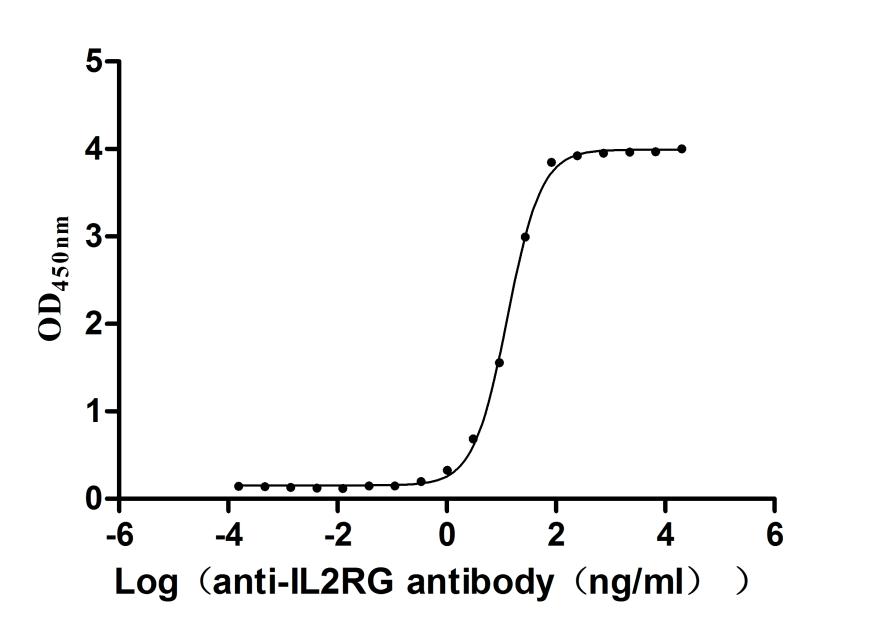
This recombinant human IL2RG protein is an active, high-purity product generated in mammalian cells to ensure proper folding and post-translational modifications essential for biological function. It comprises amino acids 23 to 254 of the human IL2RG sequence and includes a C-terminal 10xHis tag for ease of purification and detection. The recombinant IL2RG protein exhibits greater than 95% purity based on SDS-PAGE analysis. Endotoxin levels are maintained below 1.0 EU/μg, as determined by the LAL assay, making it suitable for cell-based and immunological applications. Functional testing via ELISA confirms its binding capacity. When immobilized at 2 μg/mL, IL2RG specifically interacts with the anti-IL2RG recombinant antibody (CSB-RA011651MA1HU), with an EC50 ranging from 12.19 to 13.63 ng/mL. These characteristics make it a valuable reagent for studies related to cytokine receptor signaling and immune system function.
The IL2RG protein is a component of the IL-2R complex that plays a crucial role in lymphocyte development, particularly in T and natural killer (NK) cell maturation. It is essential for the signaling of several cytokines, including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 [1][2]. Mutations or deficiencies in the IL2RG gene lead to severe combined immunodeficiency (SCID), characterized by the absence or dysfunction of T and NK cells, while B cells may be present but often lack functionality [2]. This underscores the protein's critical role in immune system function and lymphocyte proliferation.
The human IL2RG gene is located on the X chromosome and is integral to the pathophysiology of X-linked SCID, which profoundly affects immunity from infancy [2]. Individuals affected by this condition manifest an inability to mount effective immune responses, predominantly due to compromised T and NK cell development, while B cell responses may be influenced indirectly [1][2]. The common gamma chain (γc) associated with IL2RG is crucial for the transduction of vital growth and survival signals necessary for immune cell development [1][2].
The role of IL2RG in genetic models has been further elucidated through studies utilizing engineered mice, particularly the NOD-scid IL2RG null mice. These models demonstrate significant human immune cell engraftment and are employed to investigate immune system development and function under conditions that mimic SCID [1][3]. Additionally, research using knockout models has led to advancements in gene therapy approaches aimed at restoring functional immune systems in affected individuals, indicating potential pathways for therapeutic development [4][5].
Moreover, IL2RG's involvement extends into cancer biology and immunotherapy. Modulation of IL2RG signaling can influence the efficacy of immune responses in cancer treatments, making it a target for therapeutic strategies aimed at enhancing anti-tumor immunity [6]. Furthermore, its role in the formation and function of lymphocyte subtypes highlights its significance not only in immunology but also in clinical applications, including the development of gene therapies for SCID and other immunological disorders [4][5][7].
References:
[1] L. Covassin, S. Jangalwe, et al. Human immune system development and survival of non-obese diabetic (nod)-scid il2rγnull (nsg) mice engrafted with human thymus and autologous haematopoietic stem cells. Clinical & Experimental Immunology, vol. 174, no. 3, p. 372-388, 2013. https://doi.org/10.1111/cei.12180
[2] S. Suzuki, M. Iwamoto, et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell, vol. 10, no. 6, p. 753-758, 2012. https://doi.org/10.1016/j.stem.2012.04.021
[3] M. Brehm, A. Cuthbert, et al. Parameters for establishing humanized mouse models to study human immunity: analysis of human hematopoietic stem cell engraftment in three immunodeficient strains of mice bearing the il2rγnull mutation. Clinical Immunology, vol. 135, no. 1, p. 84-98, 2010. https://doi.org/10.1016/j.clim.2009.12.008
[4] M. Huston, N. Til, et al. Correction of murine scid-x1 by lentiviral gene therapy using a codon-optimized il2rg gene and minimal pretransplant conditioning. Molecular Therapy, vol. 19, no. 10, p. 1867-1877, 2011. https://doi.org/10.1038/mt.2011.127
[5] V. Poletti, S. Charrier, et al. Preclinical development of a lentiviral vector for gene therapy of x-linked severe combined immunodeficiency. Molecular Therapy — Methods & Clinical Development, vol. 9, p. 257-269, 2018. https://doi.org/10.1016/j.omtm.2018.03.002
[6] C. Jiang, J. Luo, X. Jiang, Y. Lv, & J. Dou. Predictive model of gene expression regulating invasion and migration of m2 macrophages in breast cancer: clinical prognosis and therapeutic implications. Translational Cancer Research, vol. 13, no. 8, p. 4187-4204, 2024. https://doi.org/10.21037/tcr-24-29
[7] M. Wunderlich, F. Chou, et al. Aml xenograft efficiency is significantly improved in nod/scid-il2rg mice constitutively expressing human scf, gm-csf and il-3. Leukemia, vol. 24, no. 10, p. 1785-1788, 2010. https://doi.org/10.1038/leu.2010.158