5. Signaling Pathway in Th Cell Differentiation
Many signaling pathways are involved in T cell differentiation, and the main ones are as follows:
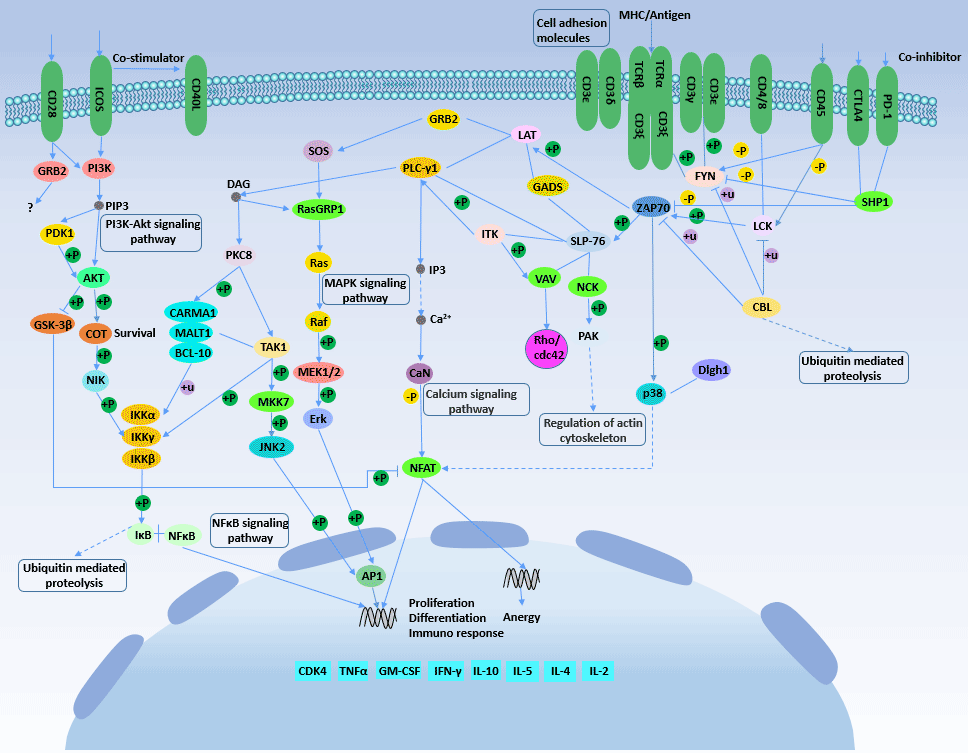
Figure 2 T cell receptor signaling pathway
T cell receptor (TCR) signaling and cytokine signaling are both essential for helper T cell differentiation. Specific TCR transcription factors such as activated T cell nuclear factor (NFATs), nuclear transcription factor-κB (NF-κB) and play a key role in T cell differentiation.
Activation of TCR can activate multiple intracellular signal transduction pathways such as mitogen activated protein kinase (MAPK), nuclear factor κB (nuclear kappa B, NF-κB), AP-1 (Fos-Jun) and CaN-NFAT.
The binding of T cell receptor (TCR) to the antigen/histocompatibility complex (MHCⅡ) on the surface of antigen presenting cells (APC) is the first signal required for T cell activation. At this stage, the factors affecting T cell differentiation are mainly the intensity of T cell acquisition signal: weak TCR activation signal can activate Ca2+ flow signal to induce IL-4 synthesis and promote T cell differentiation to Th2; Strong TCR activation signal can activate MAPK pathway to induce IFN-γ synthesis and promote T cells to differentiate to Th1.
In addition, the length of TCR triggering time also affects the differentiation of Th cells: in the presence of IL-12, a transient TCR triggers the initiation of Th1 differentiation, and a long-term TCR trigger initiates Th2 differentiation.
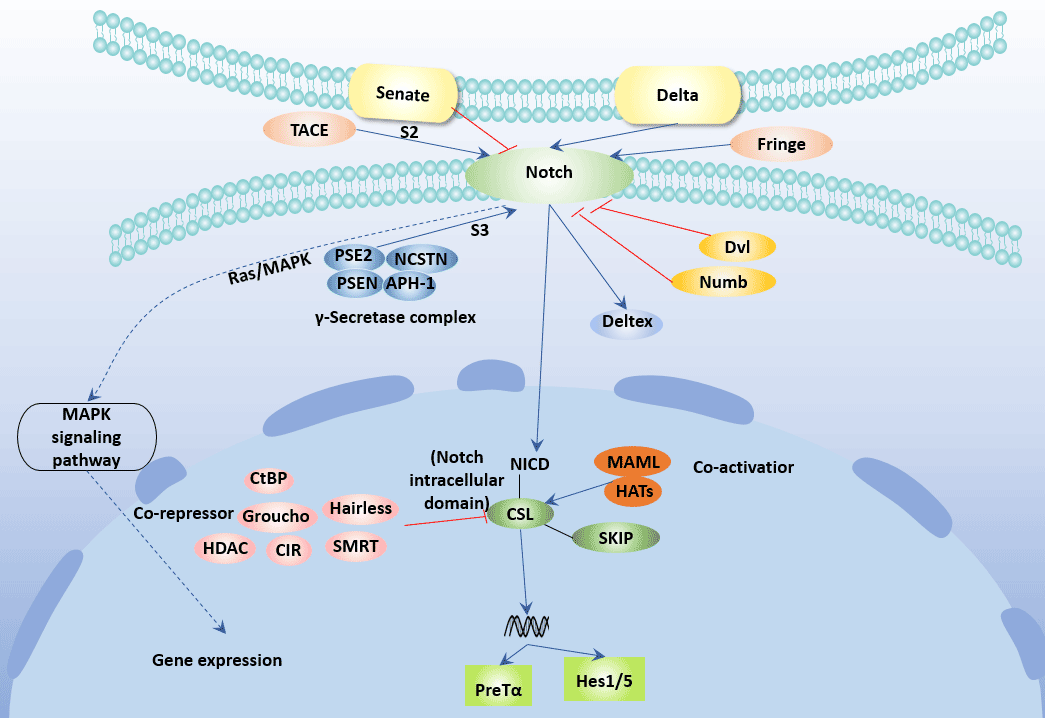
Figure 3 Notch Signaling Pathway
Currently, four Notch receptors have been found in mammals, namely Notch-1, Notch-2, Notch-3 and Notch-4. Notch ligands include the Jagged family and the Delta-like family. The Jagged family includes Jagged-l and Jagged-2, and the Delta-like family includes DLL-1, DLL-3, and DLL-4 [18] [19].
Delta-like and Jagged ligands induce polarization of T cells to Thl and Th2, respectively, which is independent of IL-4 / STAT6.
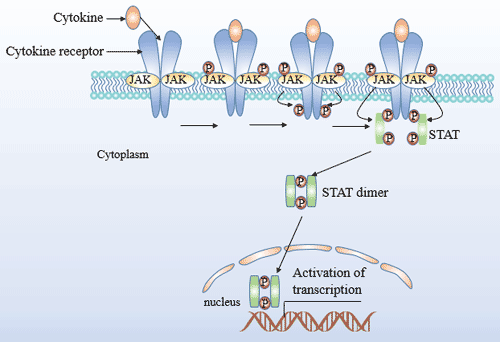
Figure 4 JAK/STAT Signaling Pathway
The JAK family protein kinase belongs to the tyrosine protein kinase and contains four members of JAK1, JAK2, JAK3 and TyK2. The STAT family contains seven members of STAT1, 2, 3, 4, 5A, 5B and 6.
JAK-STAT is a major signal transduction pathway mediated by cytokine receptors. IFN-γ activates JAK1, JAK2 and STAT1, IL-12 activates JAK2, TYK2 and STAT4, and IL-4 activates JAK1, JAK3 and STAT6. Blocking of any of the above steps can lead to inhibition of the corresponding Th cell differentiation.
Signal Transduction of Th1 Differentiation
IFN-γR and IL-12R-mediated signal transduction play an important role in the differentiation of Th1.
IFN-γR1 and IFN-γR2 were respectively bound to and tetramerized with IFN-γ. Then IFN-γR1 and IFN-γR2 are phosphorylated by JAK1 and JAK2, respectively, and STAT1 is recruited. The recruited STAT1 is phosphorylated and activated, detached from IFN-γR, dimerized and transferred into the nucleus, and binds to the GAS cis-acting element of the IFN-γ gene, thereby promoting transcription and expression of the IFN-γ gene.
IL-12R is a heterodimer composed of β1 and β2 subunits. IL-12Rβ2 is specifically expressed in Th1 cells. Upon binding of IL-12R to IL-12, JAK2 and TyK2 are activated, which in turn phosphorylates tyrosine residues in the cytoplasm of the β1 and β2 subunits. Phosphorylated β1 and β2 subunits bind to and phosphorylate STAT4. STAT4 then forms a homodimer or heterodimer with STAT3 and enters the nucleus, mediating the function of its downstream genes.
Signal Transduction of Th2 Differentiation
During the differentiation of Th2, the JAK1/3/STAT6 signal transduction pathway is mainly mediated by IL-4R and IL-13R.
JAK1/3/STAT6 pathway: IL-4 is a key cytokine that promotes Th2 cell development. The binding of IL-4 activates IL-4R, and JAK1/3 bound thereto is also phosphorylated and activated. The binding of IL-4 activates IL-4R, and JAK1/3 bound to IL-4R is also phosphorylated and activated. Phosphorylated JAK1/3 in turn phosphorylates tyrosine residues on IL-4R, phosphorylated IL-4R recruits and binds to STAT6 monomer. STAT6 monomer undergoes tyrosine phosphorylation under the action of JAK1/3 and detaches from IL-4R to form an active STAT6 dimer. STAT6 dimer nuclear translocation and further initiation of transcription and expression of IL-4 and other genes [20]. Similarly, IL-13 binds to type II IL-4R and promotes transcription and expression of many inflammatory genes via the JAK1/3/STAT6 pathway.
The MAPK signaling pathway includes four pathways: extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK), P38MAPK, and ERK5/BMK1.
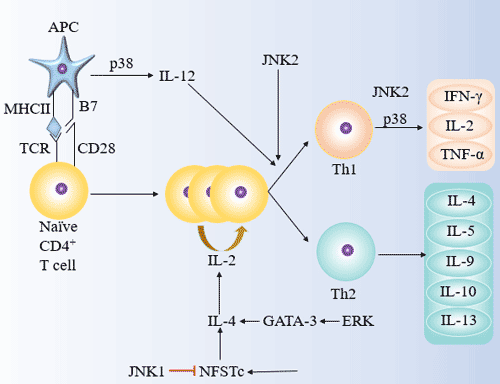
Figure 5 The roles of JNK, p38 and ERK in Th1/Th2 differentiation
The role of JNK in inducing Thl/Th2 differentiation
JNK is a stress-activated protein kinase (SAPK), including JNK1/2/3. JNK/SAPK can phosphorylate c-Jun, ATF-2 and increase its transcriptional activity, and promote the expression of c-Fos, c-Jun and ATF-2 regulatory genes.
JNK1 can inhibit the differentiation of primary CD4+ T cells into Th2, but does not affect its differentiation into Th1; JNK2 induces differentiation of CD4+ T cells into Th1 and promotes the secretion of IFN-γ in Th1 effector cells, but does not affect Th2 differentiation.
P38
The role of p38 in inducing Th1/Th2 differentiation is bidirectional. The p38MAPK pathway may induce Th1 cell differentiation by activating IFN-γ transcription. P38 can also alter the immunomodulatory function of DC by promoting the secretion of IL-12 in DC, which induces differentiation of CD4+ T cells into Th1 and mediates Th1 type immune response.
ERK
The TCR-induced Ras-ERK/MAPK pathway is capable of inducing Th2 differentiation and mediating the Th2-type immune response.
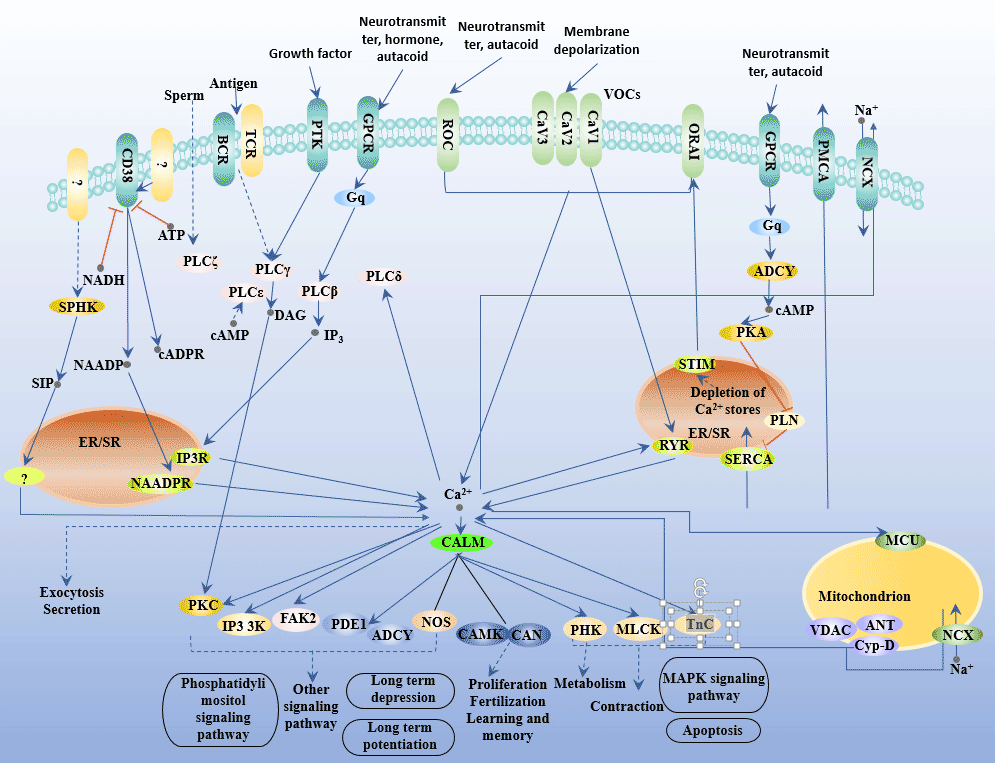
Figure 6 Calcium Signaling Pathway
Calcineurin (CaN) is a Ca2+/Calmodulin (CaM)-dependent serine/threonine phosphoprotein phosphatase that catalyzes the dephosphorylation of a variety of already phosphorylated proteins. CaN plays a role in T cell activation, differentiation and proliferation by activating the nuclear factor of activated T cell (NFAT) [21].
In T cells, Ca2+ levels regulate CaN activity, which in turn plays an important role in the activation of NFAT.
The regulation process of Ca2+-CaN-NFAT mainly has two signaling pathways: IP3-Ca2+ and DAC-PKC.
IP3 Ca2+ Signal
IP3 binds to the IP3 calcium channel in the endoplasmic reticulum, promotes the increase of cytoplasmic Ca2+ concentration, and then activates cytoplasmic CaN, which binds to NFAT and leads to its dephosphorylation and activation. After the translocation of the activated NFAT nucleus, it combines with transcription factors such as AP-1 family proteins and other activation factors to form a complex to jointly regulate the expression of cytokines.
DAC-PKC Signal
DAG activates PKC, inhibits PIP2 hydrolysis, and activates IP3 hydrolysis, so that intracellular free Ca2+ does not increase. At the same time, PKC also activates the Ca pump to reduce intracellular free Ca2+. PKC can also activate NFAT, NF-κB, AP-1 and other nuclear factors, and play a synergistic role in the activation and proliferation of lymphocytes and the production of cytokines.
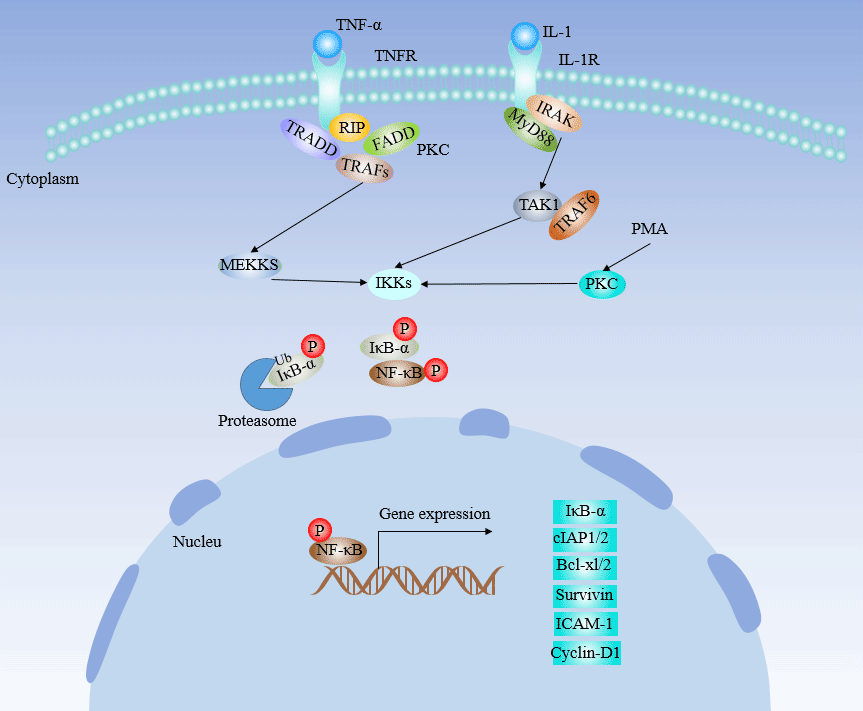
Figure 7 NF-κB Signaling Pathway
Normally, NF-κB and its inhibitor I-κB are present in the cytosol. Activation of upstream kinases (such as NF-κB-inducible kinase or MEKK1) leads to activation of I-κB kinase and phosphorylation of I-κB protein, which separates and degrades phosphorylated I-κB protein from NF-κB. Free NF-κB transfected into the nucleus to activate the target gene. The role of NF-κB in the differentiation of Th cells is just beginning. It has been found that inhibition of NF-κB activity blocks GATA-3.
The Hedgehog signaling pathway is also somewhat related to T cell differentiation. We found that the key Th2 cytokine IL-4 is a novel transcriptional target of Hh signaling in T cells, providing a mechanism for the role of Hh in Th differentiation [22].
References
[1] Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells [J]. Journal of Allergy & Clinical Immunology, 1994, 94: 1195.
[2] Romagnani S. Lymphokine Production by Human T Cells in Disease States [J]. Annual Review of Immunology, 2003, 12(12): 227-257.
[3] Valérie Dardalhon, Korn T, Kuchroo V K, et al. Role of Th1 and Th17 cells in organ-specific autoimmunity [J]. Journal of Autoimmunity, 2008, 31(3): 0-256.
[4] Wan Y Y, Flavell R A. How Diverse-CD4 Effector T Cells and their Functions [J]. Journal of Molecular Cell Biology, 2009, 1(1): 20-36.
[5] Murphy K M, Ouyang W, Farrar J D, et al. Signaling and Transcription in T Helper Development [J]. Annual Review of Immunology, 2000, 18(1): 451-494.
[6] Huang Z, Xin J, Coleman J, et al. IFN-γ Suppresses STAT6 Phosphorylation by Inhibiting Its Recruitment to the IL-4 Receptor [J]. The Journal of Immunology, 2005, 174(3): 1332-1337.
[7] Gately M K, Renzetti L M, Magram J, et al. THE INTERLEUKIN-12/INTERLEUKIN-12-RECEPTOR SYSTEM: Role in Normal and Pathologic Immune Responses [J]. Annual Review of Immunology, 1998, 16(1): 495-521.
[8] Glimcher L H, Murphy K M D A J. Lineage commitment in the immune system: the T helper lymphocyte grows up [J]. Genes Dev, 2000, 14(14): 1693-1711.
[9] Ansel K M, Greenwald R J, Agarwal S, et al. Deletion of a conserved IL-4 silencer impairs T helper type 1-mediated immunity [J]. Nature Immunology, 2004, 5(12): 1251-1259.
[10] O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets [J]. Immunity, 1998, 8(3): 275.
[11] Das J, Chen C H, Yang L, et al. A critical role for NF-kappa B in GATA3 expression and TH2 differentiation in allergic airway inflammation [J]. Nature Immunology, 2001, 2(1): 45-50.
[12] Losman J A. SOCS-1 is a potent inhibitor of IL-4 signal transduction [J]. J. Immunol. 1999, 162(7): 3770-3774.
[13] Farrar J D, Asnagli H, Murphy K M. T helper subset development: roles of instruction, selection, and transcription [J]. Journal of Clinical Investigation, 2002, 109(4): 31-5.
[14] Szabo S J, Kim S T, Costa G L, et al. A Novel Transcription Factor, T-bet, Directs Th1 Lineage Commitment [J]. CELL, 2000, 100(6): 0-669.
[15] Mullen A C, High F A, Hutchins A S, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection [J]. Science, 2001, 292(5523): 1907-1910.
[16] Djuretic I M, Levanon D, Negreanu V, et al. Erratum: Transcription factors T-bet and Runx3 cooperate to activate IFN-γ and silence IL-4 in T helper type 1 cells (Nature Immunology) [J]. Nature Immunology, 2007, 8(2): 145-153.
[17] Zhang D H, Cohn L, Ray P, et al. Transcription Factor GATA-3 Is Differentially Expressed in Murine Th1 and Th2 Cells and Controls Th2-specific Expression of the Interleukin-5 Gene [J]. Journal of Biological Chemistry, 1997, 272(34): 21597-21603.
[18] Sara González-García, Marina García-Peydró, Alcain J, et al. Notch1 and IL-7 Receptor Signalling in Early T-cell Development and Leukaemia [J]. Current Topics in Microbiology & Immunology, 2012, 360: 47.
[19] Greenwald I, Kovall R. Notch signaling: genetics and structure [J]. Wormbook the Online Review of C Elegans Biology, 2013: 1.
[20] Rosenwasser L J, Zimmermann N, Hershey G K, et al. Chemokines in asthma: Cooperative interaction between chemokines and IL-13 [J]. Journal of Allergy & Clinical Immunology, 2003, 111(2): 227-242.
[21] Gwack Y, Feske S, Srikanth S, et al. Signalling to transcription: Store-operated Ca2+ entry and NFAT activation in lymphocytes [J]. Cell Calcium, 2007, 42(2): 145-156.
[22] Furmanski A L, Saldana J I, Ono M, et al. Tissue-Derived Hedgehog Proteins Modulate Th Differentiation and Disease [J]. The Journal of Immunology, 2013, 190(6): 2641-2649.










Comments
Leave a Comment