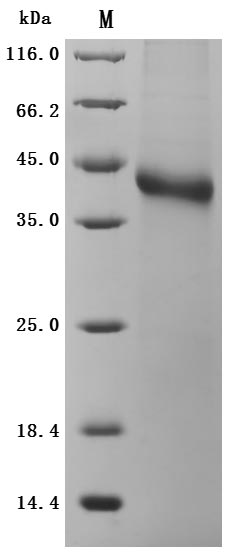
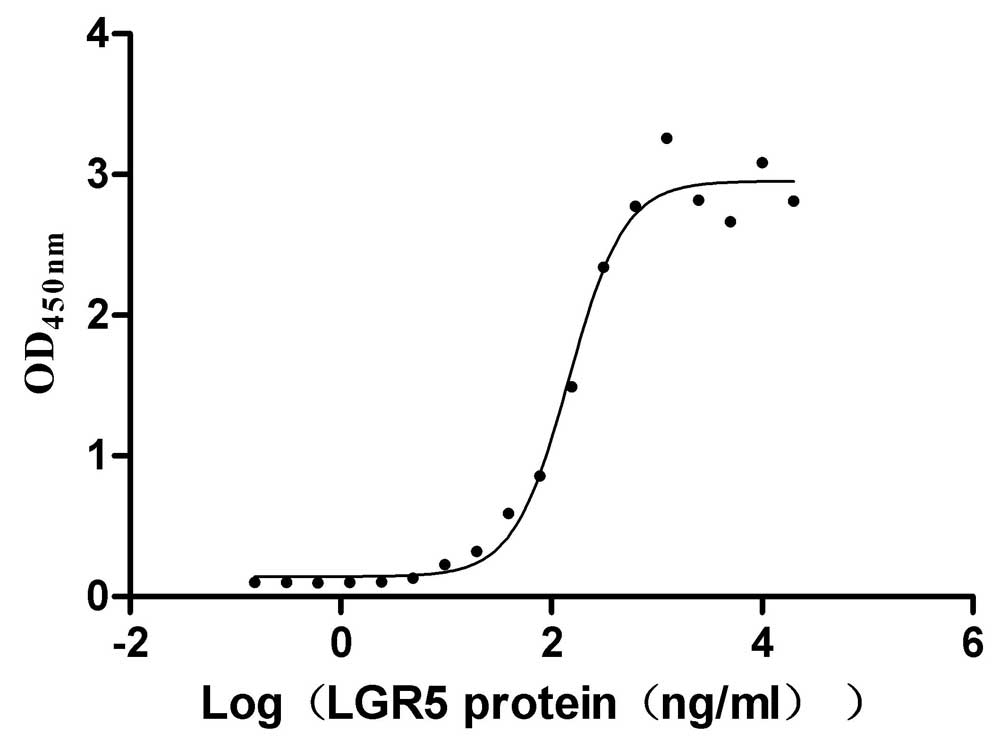
The recombinant human RSPO1 protein is expressed in mammalian cells. Its expression region corresponds to the 31-263aa of the human RSPO1. It is fused with a 10xHis-Avi-tag at the C-terminus. The resulting recombinant RSPO1 protein is extracted after cell lysis and purified with affinity chromatography. SDS-PAGE confirms its purity above 95%, and the LAL method ensures its endotoxin levels are below 1.0 EU/μg. It is validated to be an active protein. Functional ELISA validates its binding to the human LGR5 (CSB-MP012906HU1), with an EC50 of 124.0-174.1 ng/mL.
Human RSPO1 is a member of the R-spondin family of secreted proteins, which play critical roles in enhancing Wnt signaling pathways, particularly the Wnt/β-catenin pathway. RSPO1 is known for its ability to stabilize β-catenin, a key effector in Wnt signaling, thereby promoting various biological processes including cell proliferation, differentiation, and tissue homeostasis [1][2]. RSPO1 is characterized by two furin-like repeats and a thrombospondin type 1 repeat, which are essential for its signaling activity [3][4].
RSPO1 is expressed in various tissues, including the intestine and gonads, and has been implicated in developmental processes such as ovarian development and nephron progenitor maintenance [5][6][7]. Its expression is particularly significant during early ovarian development, where it augments β-catenin signaling, suggesting a role in sexual differentiation [5][7]. Furthermore, mutations in the RSPO1 gene have been associated with disorders of sex development, including cases of male-to-female sex reversal [8][9].
RSPO1 has been shown to influence keratinocyte proliferation and differentiation, making it relevant in the context of skin cancers such as squamous cell carcinoma [10][11]. Moreover, RSPO1's interaction with other proteins, such as LGR4 and LGR5, facilitates its signaling capabilities, linking it to various cellular responses and potentially to tumorigenesis [11][12].
Recent studies have also explored the role of RSPO1 in metabolic processes, particularly its association with obesity and insulin resistance [13][14]. Additionally, RSPO1 has been identified as a protective factor against radiation-induced gastrointestinal damage, further emphasizing its significance in tissue repair and regeneration [15][16].
References:
[1] M. Tsuchiya, Y. Niwa, & S. Simizu. N-glycosylation of r-spondin1 at asn137 negatively regulates its secretion and wnt/β-catenin signaling-enhancing activity, Oncology Letters, vol. 11, no. 5, p. 3279-3286, 2016. https://doi.org/10.3892/ol.2016.4425
[2] O. Elkady. R-spondin-1 level in different dermatoses: a comprehensive review, Benha Journal of Applied Sciences, vol. 8, no. 2, p. 25-29, 2023. https://doi.org/10.21608/bjas.2023.216091.1186
[3] S. Tomaselli, F. Megiorni, C. Bernardo, A. Felici, G. Marrocco, G. Maggiulli, et al. Syndromic true hermaphroditism due to an r-spondin1 (rspo1) homozygous mutation, Human Mutation, vol. 29, no. 2, p. 220-226, 2008. https://doi.org/10.1002/humu.20665
[4] Y. Xie, R. Zamponi, O. Charlat, M. Ramones, S. Swalley, X. Jiang, et al. Interaction with both znrf3 and lgr4 is required for the signalling activity of r‐spondin, Embo Reports, vol. 14, no. 12, p. 1120-1126, 2013. https://doi.org/10.1038/embor.2013.167
[5] S. Tomaselli, F. Megiorni, L. Lin, M. Mazzilli, D. Gerrelli, S. Majore, et al. Human rspo1/r-spondin1 is expressed during early ovary development and augments β-catenin signaling, Plos One, vol. 6, no. 1, p. e16366, 2011. https://doi.org/10.1371/journal.pone.0016366
[6] V. Vidal, F. Motamedi, S. Rekima, E. Gregoire, E. Szenker‐Ravi, M. Leushacke, et al. R-spondin signalling is essential for the maintenance and differentiation of mouse nephron progenitors, Elife, vol. 9, 2020. https://doi.org/10.7554/elife.53895
[7] C. Smith, C. Shoemaker, K. Roeszler, J. Queen, D. Crews, & A. Sinclair. Cloning and expression of r-spondin1in different vertebrates suggests a conserved role in ovarian development, BMC Developmental Biology, vol. 8, no. 1, 2008. https://doi.org/10.1186/1471-213x-8-72
[8] M. Volleth and P. Wieacker. WNT4 and RSPO1 are not involved in a case of male-to-female sex reversal with partial duplication of 1p, Sexual Development, vol. 1, no. 2, p. 111-113, 2007. https://doi.org/10.1159/000100032
[9] K. Tallapaka, V. Venugopal, A. Dalal, & S. Aggarwal. Novel rspo1 mutation causing 46,xx testicular disorder of sex development with palmoplantar keratoderma: a review of literature and expansion of clinical phenotype, American Journal of Medical Genetics Part A, vol. 176, no. 4, p. 1006-1010, 2018. https://doi.org/10.1002/ajmg.a.38646
[10] E. Choi, J. Yun, E. Jeon, H. Won, Y. Ko, & S. Kim. Prognostic significance of rspo1, wnt1, p16, wt1, and sdc1 expressions in invasive ductal carcinoma of the breast, World Journal of Surgical Oncology, vol. 11, no. 1, 2013. https://doi.org/10.1186/1477-7819-11-314
[11] A. Glinka, C. Dolde, N. Kirsch, Y. Huang, O. Kazanskaya, D. Ingelfinger, et al. Lgr4 and lgr5 are r‐spondin receptors mediating wnt/β‐catenin and wnt/pcp signalling, Embo Reports, vol. 12, no. 10, p. 1055-1061, 2011. https://doi.org/10.1038/embor.2011.175
[12] X. Gong, K. Carmon, Q. Lin, A. Thomas, J. Yi, & Q. Liu. Lgr6 is a high affinity receptor of r-spondins and potentially functions as a tumor suppressor, Plos One, vol. 7, no. 5, p. e37137, 2012. https://doi.org/10.1371/journal.pone.0037137
[13] Y. Kang, J. Kim, H. Yi, K. Joung, H. Kim, & B. Ku. Serum r-spondin 1 is a new surrogate marker for obesity and insulin resistance, Diabetes & Metabolism Journal, vol. 43, no. 3, p. 368, 2019. https://doi.org/10.4093/dmj.2018.0066
[14] O. BAŞPINAR>, Y. Simsek, D. Kocer, O. Dizdar, & H. Does Serum R-Spondin-1 Play a Role in PCOS Pathophysiology? Genel Tıp Dergisi, vol. 32, no. 5, p. 490-493, 2022. https://doi.org/10.54005/geneltip.1111079
[15] P. Bhanja, S. Saha, R. Kabarriti, L. Liu, N. Roy‐Chowdhury, J. Roy‐Chowdhury, et al. Protective role of r-spondin1, an intestinal stem cell growth factor, against radiation-induced gastrointestinal syndrome in mice, Plos One, vol. 4, no. 11, p. e8014, 2009. https://doi.org/10.1371/journal.pone.0008014
[16] J. Zhao, K. Kim, J. Vera, S. Palencia, M. Wagle, & A. Abo. R-spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical wnt/β-catenin pathway, Proceedings of the National Academy of Sciences, vol. 106, no. 7, p. 2331-2336, 2009. https://doi.org/10.1073/pnas.0805159106